Discovery and Development of 5-[(5S,9R)-9- (4-Cyanophenyl)-3-(3,5-dichlorophenyl)-1- methyl-2,4-dioxo-1,3,7-triazaspiro[4.4]non- 7-yl-methyl]-3-thiophenecarboxylic acid (BMS-587101)-A Small Molecule Antagonist Leukocyte Function Associated Antigen-1.
Potin, D., Launay, M., Monatlik, F., Malabre, P., Fabreguettes, M., Fouquet, A., Maillet, M., Nicolai, E., Dorgeret, L., Chevallier, F., Besse, D., Dufort, M., Caussade, F., Ahmad, S.Z., Stetsko, D.K., Skala, S., Davis, P.M., Balimane, P., Patel, K., Yang, Z., Marathe, P., Postelneck, J., Townsend, R.M., Goldfarb, V., Sheriff, S., Einspahr, H., Kish, K., Malley, M.F., Dimarco, J.D., Gougoutas, J.Z., Kadiyala, P., Cheney, D.L., Tejwani, R.W., Murphy, D.K., McIntyre, K.W., Yang, X., Chao, S., Leith, L., Xiao, Z., Mathur, A., Chen, B.C., Wu, D.R., Traeger, S.C., McKinnon, M., Barrish, J.C., Robl, J.A., Iwanowicz, E.J., Suchard, S.J., Dhar, T.G.(2006) J Med Chem 49: 6946-6949
- PubMed: 17125246
- DOI: https://doi.org/10.1021/jm0610806
- Primary Citation of Related Structures:
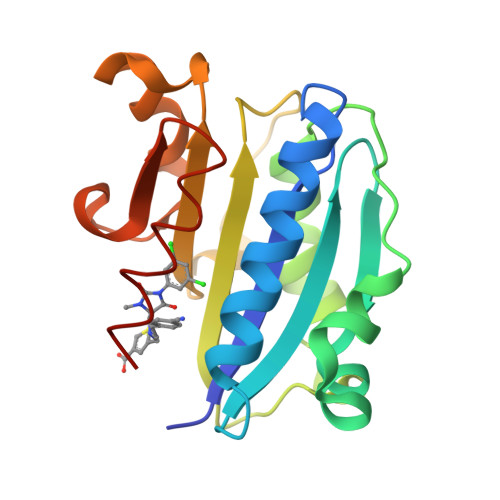
2ICA - PubMed Abstract:
LFA-1 (leukocyte function-associated antigen-1), is a member of the beta2-integrin family and is expressed on all leukocytes. This letter describes the discovery and preliminary SAR of spirocyclic hydantoin based LFA-1 antagonists that culminated in the identification of analog 8 as a clinical candidate. We also report the first example of the efficacy of a small molecule LFA-1 antagonist in combination with CTLA-4Ig in an animal model of transplant rejection.
Organizational Affiliation:
Cerep, 19 avenue du Quebec, 91951 Courtaboeuf cedex, France.