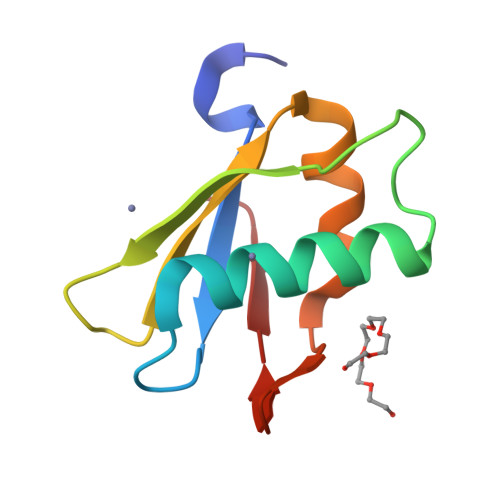
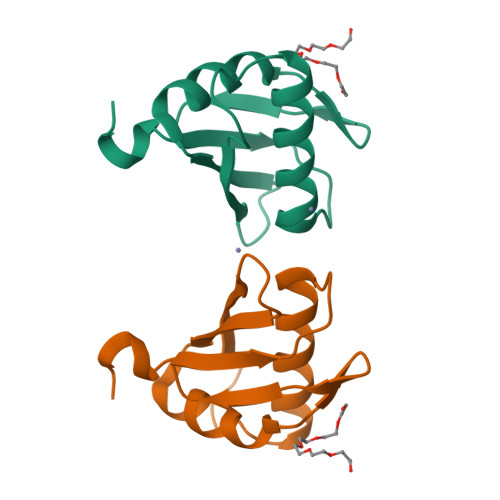
Alternative Conformations at the RNA-binding Surface of the N-terminal U2AF(65) RNA Recognition Motif.
Thickman, K.R., Sickmier, E.A., Kielkopf, C.L.(2007) J Mol Biology 366: 703-710
- PubMed: 17188295
- DOI: https://doi.org/10.1016/j.jmb.2006.11.077
- Primary Citation of Related Structures:
2HZC - PubMed Abstract:
The essential pre-mRNA splicing factor, U2 auxiliary factor 65KD (U2AF(65)) recognizes the polypyrimidine tract (Py-tract) consensus sequence of the pre-mRNA using two RNA recognition motifs (RRMs), the most prevalent class of eukaryotic RNA-binding domain. The Py-tracts of higher eukaryotic pre-mRNAs are often interrupted with purines, yet U2AF(65) must identify these degenerate Py-tracts for accurate pre-mRNA splicing. Previously, the structure of a U2AF(65) variant in complex with poly(U) RNA suggested that rearrangement of flexible side-chains or bound water molecules may contribute to degenerate Py-tract recognition by U2AF(65). Here, the X-ray structure of the N-terminal RRM domain of U2AF(65) (RRM1) is described at 1.47 A resolution in the absence of RNA. Notably, RNA-binding by U2AF(65) selectively stabilizes pre-existing alternative conformations of three side-chains located at the RNA interface (Arg150, Lys225, and Arg227). Additionally, a flexible loop connecting the beta2/beta3 strands undergoes a conformational change to interact with the RNA. These pre-existing alternative conformations may contribute to the ability of U2AF(65) to recognize a variety of Py-tract sequences. This rare, high-resolution view of an important member of the RRM class of RNA-binding domains highlights the role of alternative side-chain conformations in RNA recognition.
Organizational Affiliation:
Department of Biochemistry and Molecular Biology, Johns Hopkins University Bloomberg School of Public Health, Baltimore, MD 21205, USA.