NikR-operator complex structure and the mechanism of repressor activation by metal ions.
Schreiter, E.R., Wang, S.C., Zamble, D.B., Drennan, C.L.(2006) Proc Natl Acad Sci U S A 103: 13676-13681
- PubMed: 16945905
- DOI: https://doi.org/10.1073/pnas.0606247103
- Primary Citation of Related Structures:
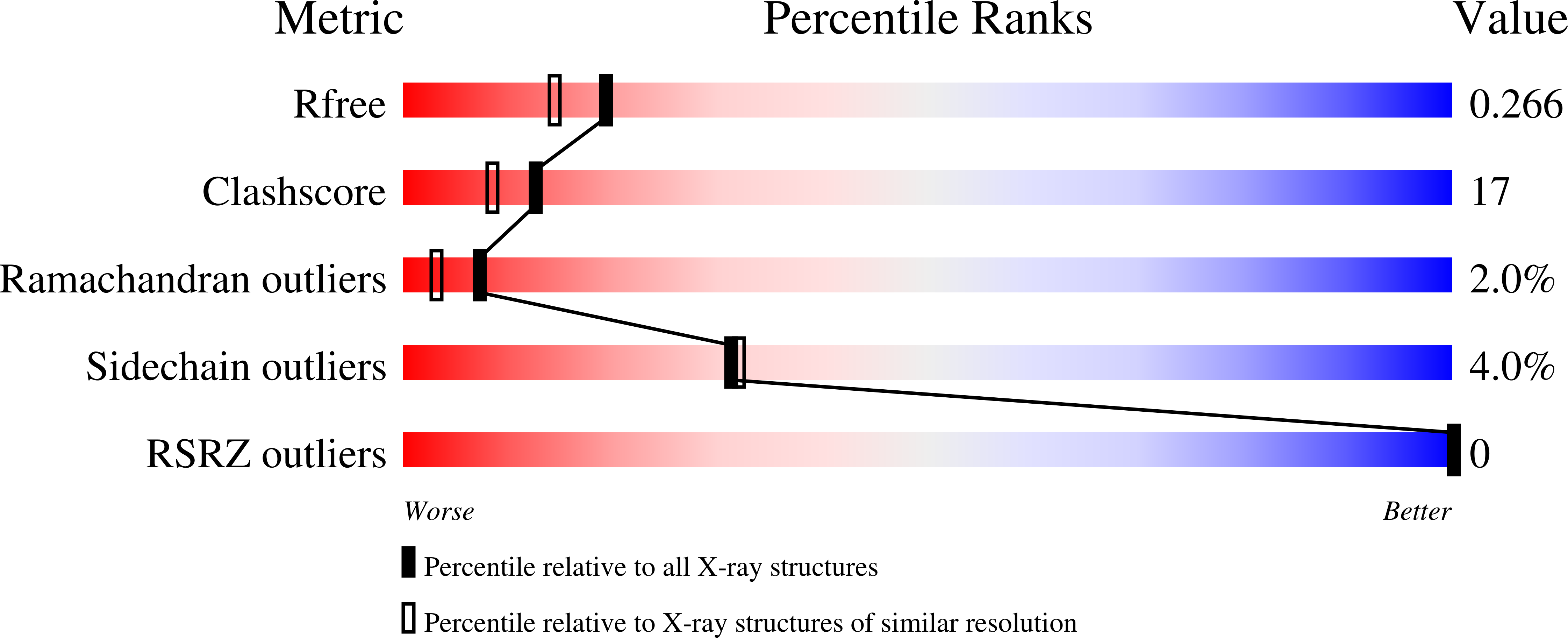
2HZA, 2HZV - PubMed Abstract:
Metal ion homeostasis is critical to the survival of all cells. Regulation of nickel concentrations in Escherichia coli is mediated by the NikR repressor via nickel-induced transcriptional repression of the nickel ABC-type transporter, NikABCDE. Here, we report two crystal structures of nickel-activated E. coli NikR, the isolated repressor at 2.1 A resolution and in a complex with its operator DNA sequence from the nik promoter at 3.1 A resolution. Along with the previously published structure of apo-NikR, these structures allow us to evaluate functional proposals for how metal ions activate NikR, delineate the drastic conformational changes required for operator recognition, and describe the formation of a second metal-binding site in the presence of DNA. They also provide a rare set of structural views of a ligand-responsive transcription factor in the unbound, ligand-induced, and DNA-bound states, establishing a model system for the study of ligand-mediated effects on transcription factor function.
Organizational Affiliation:
Department of Chemistry, Massachusetts Institute of Technology, Cambridge, MA 02139, USA.