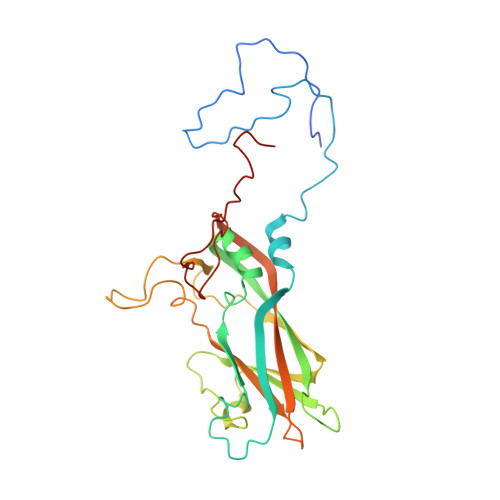
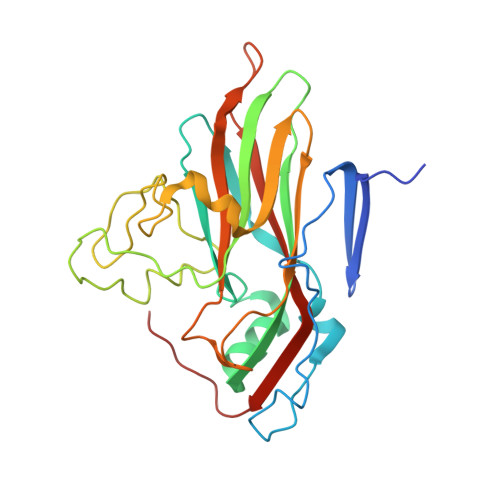
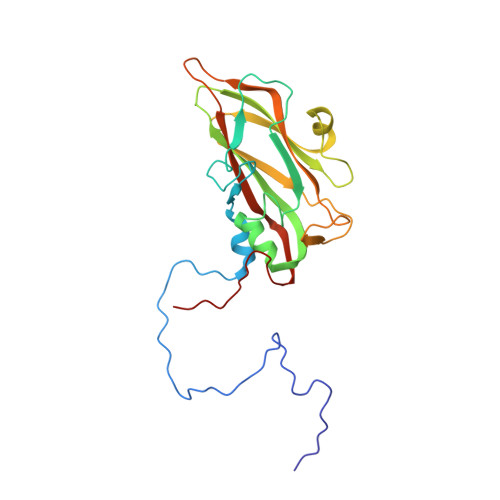
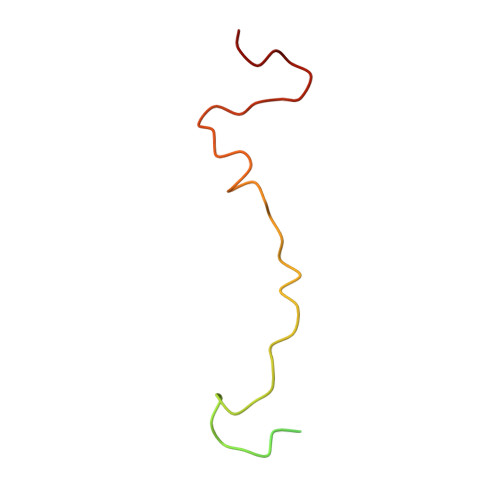
A comparison of the anti-rhinoviral drug binding pocket in HRV14 and HRV1A.
Kim, K.H., Willingmann, P., Gong, Z.X., Kremer, M.J., Chapman, M.S., Minor, I., Oliveira, M.A., Rossmann, M.G., Andries, K., Diana, G.D., Dutko, F.J., McKinlay, M.A., Pevear, D.C.(1993) J Mol Biol 230: 206-227
- PubMed: 8383771
- DOI: https://doi.org/10.1006/jmbi.1993.1137
- Primary Citation of Related Structures:
2HWB, 2HWC, 2HWD, 2HWE, 2HWF - PubMed Abstract:
The three-dimensional structures of two human rhinovirus serotypes (HRV14 and HRV1A) are compared when complexed with various antiviral agents. Although these agents all bind into the same hydrophobic pocket, the exact viral-drug interactions differ. In the absence of drugs, the pocket is occupied by a fatty acid in HRV1A, but is empty in HRV14 except for two water molecules. The conformation of each drug is dependent upon the shape of the hydrophobic pocket. In HRV14 the major residues determining the shape of the binding site are Y1128, P1174 and M1224, corresponding to I1125, M1169 and I1220 in HRV1A. When there is no cofactor or a drug in the pocket, the entrance to the pocket is open. However, the entrance is closed when the pocket is occupied by a cofactor or a drug. There are relatively small conformational changes when the agents displace the natural cofactor in HRV1A. In contrast, there are much larger conformational changes on binding a drug in HRV14. These differences cause an inhibition of viral attachment in HRV14 but not in HRV1A. Binding of the drugs results in three additional interprotomer hydrogen bonds in HRV14 and one in HRV1A. These hydrogen bonds and a potential loss of flexibility upon efficient packing of the pocket may contribute to the inhibition of uncoating in both serotypes.
Organizational Affiliation:
Department of Biological Sciences, Purdue University West Lafayette, IN 47907.