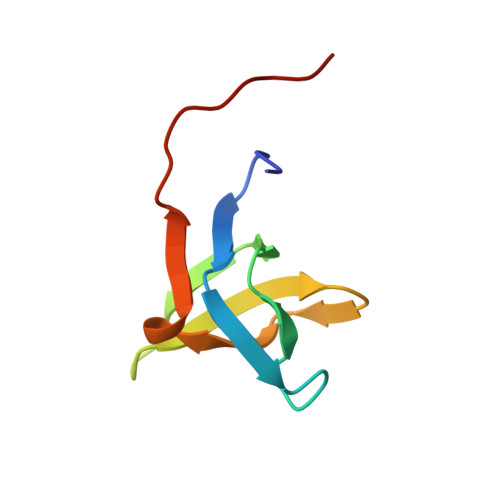
Solution structure of the SH3 domain of phospholipase C-gamma.
Kohda, D., Hatanaka, H., Odaka, M., Mandiyan, V., Ullrich, A., Schlessinger, J., Inagaki, F.(1993) Cell 72: 953-960
- PubMed: 7681365
- DOI: https://doi.org/10.1016/0092-8674(93)90583-c
- Primary Citation of Related Structures:
1HSQ, 2HSP - PubMed Abstract:
SH3 (Src homology 3) domains are found in many signaling proteins and appear to function as binding modules for cytoplasmic target proteins. The solution structure of the SH3 domain of human phospholipase C-gamma (PLC-gamma) was determined by two-dimensional 1H NMR analysis. This SH3 domain is composed of eight antiparallel beta strands consisting of two successive "Greek key" motifs, which form a barrel-like structure. The conserved aliphatic and aromatic residues form a hydrophobic pocket on the molecular surface, and the conserved carboxylic residues are localized to the periphery. The hydrophobic pocket may serve as a binding site for target proteins. Analysis of the slowly exchanging amide protons by NMR measurements indicates that despite containing a high content of beta structure, the SH3 domain of PLC-gamma is flexible.
Organizational Affiliation:
Department of Molecular Physiology, Tokyo Metropolitan Institute of Medical Science, Japan.