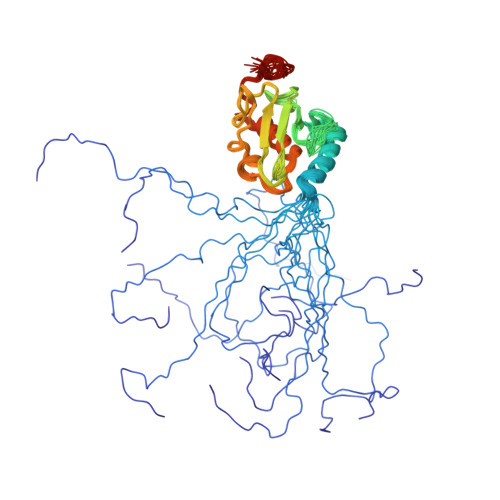
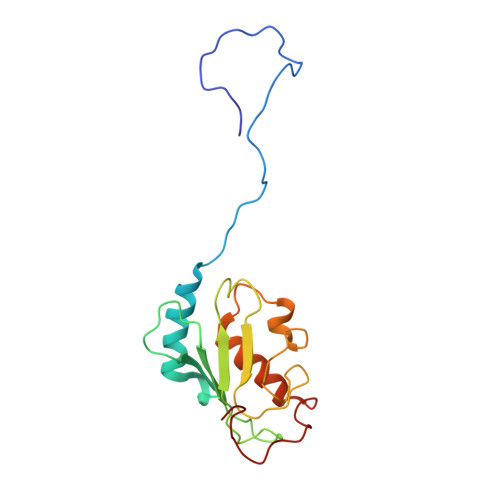
Structure and interactions of the ubiquitin-conjugating enzyme variant human uev1a: implications for enzymatic synthesis of polyubiquitin chains(,).
Hau, D.D., Lewis, M.J., Saltibus, L.F., Pastushok, L., Xiao, W., Spyracopoulos, L.(2006) Biochemistry 45: 9866-9877
- PubMed: 16893187
- DOI: https://doi.org/10.1021/bi060631r
- Primary Citation of Related Structures:
2HLW - PubMed Abstract:
Lys(63)-linked polyubiquitination of TRAF2 or TRAF6 is an essential step within the signal transduction cascade responsible for activation of p38, c-Jun N-terminal kinase, and the transcription factor NF-kappaB. Attachment of ubiquitin (Ub) to a TRAF, and conjugation of Ub molecules to form a polyUb chain, is catalyzed by a heterodimer composed of a catalytically active E2 (hUbc13), involved in covalent bond transfer, and hUev1a, an E2-like protein involved in substrate Ub binding. Given the key biochemical processes in which hUev1a is involved, it is important to determine the molecular basis of the catalytic mechanism for Lys(63)-linked protein ubiquitination. Nuclear magnetic resonance (NMR) spectroscopy was used to determine the structure of hUev1a and its interactions with Ub and hUbc13. A structural model for the Ub-hUev1a-hUbc13-Ub tetramer was developed to gain chemical insight into the synthesis of Lys(63)-linked Ub chains. We propose that a network of hydrogen bonds involving hUbc13-Asp(81) and Ub-Glu(64) positions Ub-Lys(63) proximal to the active site. Interestingly, restrained molecular dynamics simulations in implicit solvent indicate that deprotonation of Ub-Lys(63) does not involve a general Asp or Glu base and may occur when the amino group approaches the thioester carbonyl carbon near the Bürgi-Dunitz trajectory.
Organizational Affiliation:
Department of Biochemistry, University of Alberta, Edmonton, Alberta T6G 2H7, Canada.