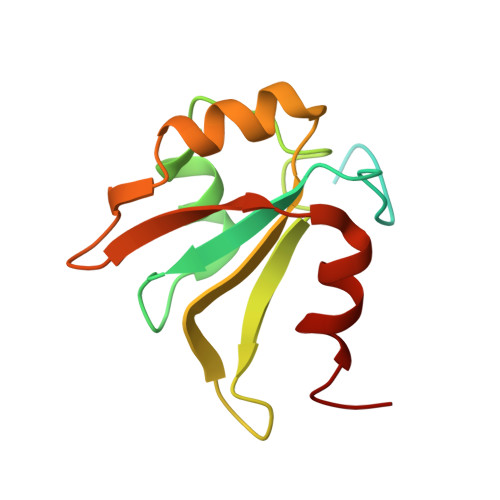
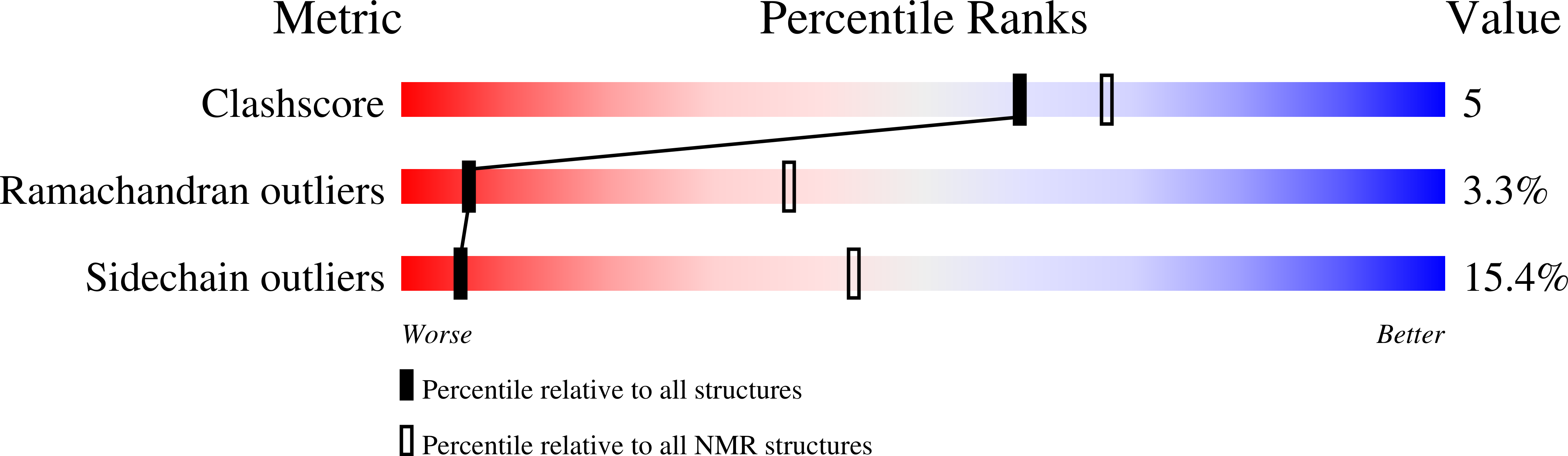NMR structure of the three quasi RNA recognition motifs (qRRMs) of human hnRNP F and interaction studies with Bcl-x G-tract RNA: a novel mode of RNA recognition.
Dominguez, C., Allain, F.H.(2006) Nucleic Acids Res 34: 3634-3645
- PubMed: 16885237
- DOI: https://doi.org/10.1093/nar/gkl488
- Primary Citation of Related Structures:
2HGL, 2HGM, 2HGN - PubMed Abstract:
The heterogeneous nuclear ribonucleoprotein (hnRNP) F belongs to the hnRNP H family involved in the regulation of alternative splicing and polyadenylation and specifically recognizes poly(G) sequences (G-tracts). In particular, hnRNP F binds a G-tract of the Bcl-x RNA and regulates its alternative splicing, leading to two isoforms, Bcl-x(S) and Bcl-x(L), with antagonist functions. In order to gain insight into G-tract recognition by hnRNP H members, we initiated an NMR study of human hnRNP F. We present the solution structure of the three quasi RNA recognition motifs (qRRMs) of hnRNP F and identify the residues that are important for the interaction with the Bcl-x RNA by NMR chemical shift perturbation and mutagenesis experiments. The three qRRMs exhibit the canonical betaalphabetabetaalphabeta RRM fold but additional secondary structure elements are present in the two N-terminal qRRMs of hnRNP F. We show that qRRM1 and qRRM2 but not qRRM3 are responsible for G-tract recognition and that the residues of qRRM1 and qRRM2 involved in G-tract interaction are not on the beta-sheet surface as observed for the classical RRM but are part of a short beta-hairpin and two adjacent loops. These regions define a novel interaction surface for RNA recognition by RRMs.
Organizational Affiliation:
Institute of Molecular Biology and Biophysics, ETH Zürich, CH-8093 Zürich, Switzerland.