Structural and Functional Analysis of a Conjugated Bile Salt Hydrolase from Bifidobacterium longum Reveals an Evolutionary Relationship with Penicillin V Acylase.
Kumar, R.S., Brannigan, J.A., Prabhune, A.A., Pundle, A.V., Dodson, G.G., Dodson, E.J., Suresh, C.G.(2006) J Biol Chem 281: 32516-32525
- PubMed: 16905539
- DOI: https://doi.org/10.1074/jbc.M604172200
- Primary Citation of Related Structures:
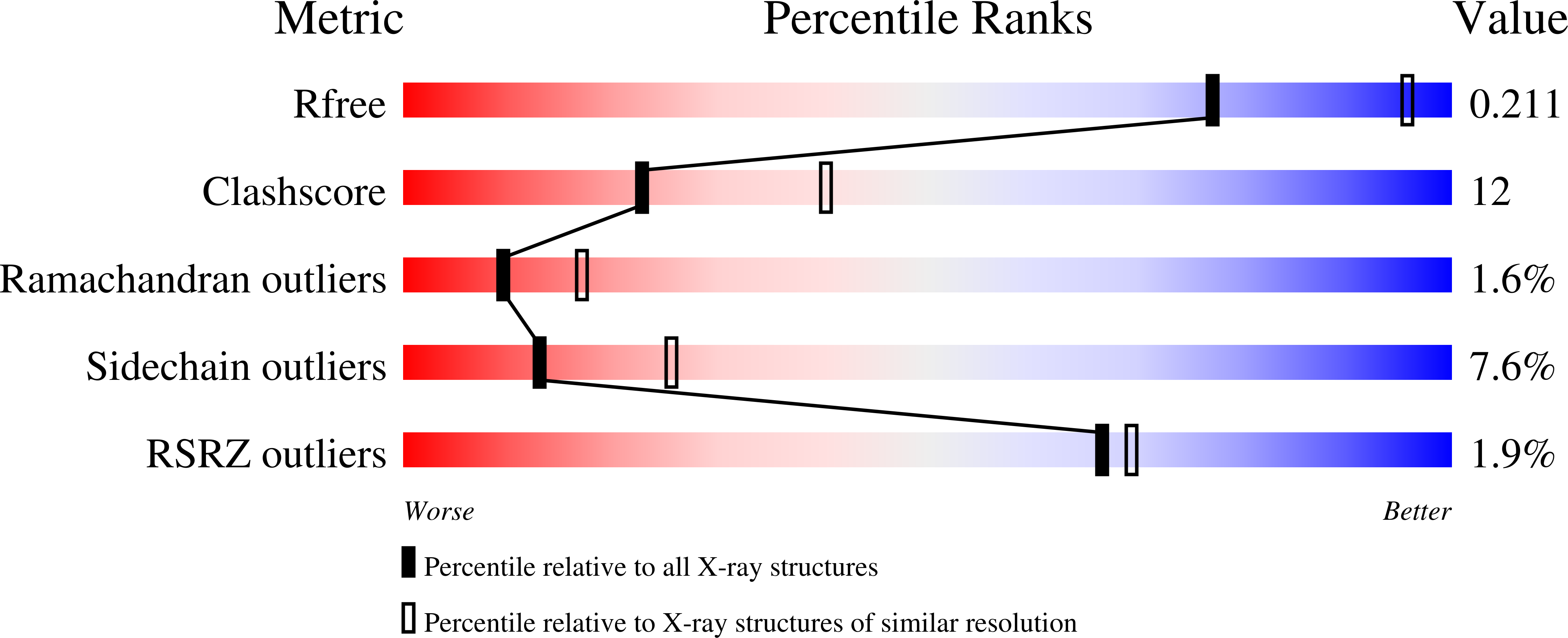
2HEZ, 2HF0 - PubMed Abstract:
Bile salt hydrolase (BSH) is an enzyme produced by the intestinal microflora that catalyzes the deconjugation of glycine- or taurine-linked bile salts. The crystal structure of BSH reported here from Bifidobacterium longum reveals that it is a member of N-terminal nucleophil hydrolase structural superfamily possessing the characteristic alphabetabetaalpha tetra-lamellar tertiary structure arrangement. Site-directed mutagenesis of the catalytic nucleophil residue, however, shows that it has no role in zymogen processing into its corresponding active form. Substrate specificity was studied using Michaelis-Menten and inhibition kinetics and fluorescence spectroscopy. These data were compared with the specificity profile of BSH from Clostridium perfrigens and pencillin V acylase from Bacillus sphaericus, for both of which the three-dimensional structures are available. Comparative analysis shows a gradation in activity toward common substrates, throwing light on a possible common route toward the evolution of pencillin V acylase and BSH.
Organizational Affiliation:
Division of Biochemical Sciences, National Chemical Laboratory, Pune 411 008, India.