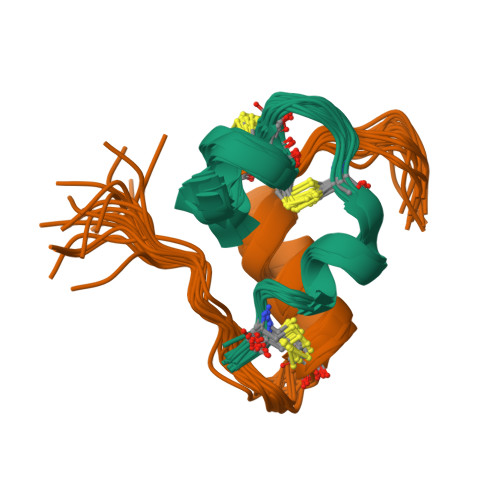
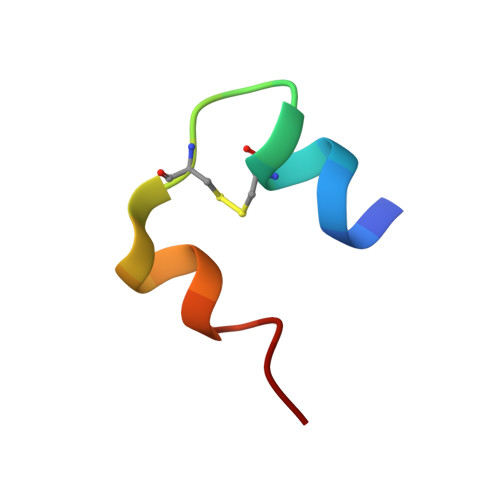
A Conserved Histidine in Insulin Is Required for the Foldability of Human Proinsulin: Structure and function of an Alab5 analog.
Hua, Q.X., Liu, M., Hu, S.Q., Jia, W., Arvan, P., Weiss, M.A.(2006) J Biological Chem 281: 24889-24899
- PubMed: 16728398
- DOI: https://doi.org/10.1074/jbc.M602617200
- Primary Citation of Related Structures:
2H67 - PubMed Abstract:
The insulins of eutherian mammals contain histidines at positions B5 and B10. The role of His(B10) is well defined: although not required in the mature hormone for receptor binding, in the islet beta cell this side chain functions in targeting proinsulin to glucose-regulated secretory granules and provides axial zincbinding sites in storage hexamers. In contrast, the role of His(B5) is less well understood. Here, we demonstrate that its substitution with Ala markedly impairs insulin chain combination in vitro and blocks the folding and secretion of human proinsulin in a transfected mammalian cell line. The structure and stability of an Ala(B5)-insulin analog were investigated in an engineered monomer (DKP-insulin). Despite its impaired foldability, the structure of the Ala(B5) analog retains a native-like T-state conformation. At the site of substitution, interchain nuclear Overhauser effects are observed between the methyl resonance of Ala(B5) and side chains in the A chain; these nuclear Overhauser effects resemble those characteristic of His(B5) in native insulin. Substantial receptor binding activity is retained (80 +/- 10% relative to the parent monomer). Although the thermodynamic stability of the Ala(B5) analog is decreased (DeltaDeltaG(u) = 1.7 +/- 0.1 kcal/mol), consistent with loss of His(B5)-related interchain packing and hydrogen bonds, control studies suggest that this decrement cannot account for its impaired foldability. We propose that nascent long-range interactions by His(B5) facilitate alignment of Cys(A7) and Cys(B7) in protein-folding intermediates; its conservation thus reflects mechanisms of oxidative folding rather than structure-function relationships in the native state.
Organizational Affiliation:
Department of Biochemistry, Case Western Reserve University, Cleveland, Ohio 44106-4935.