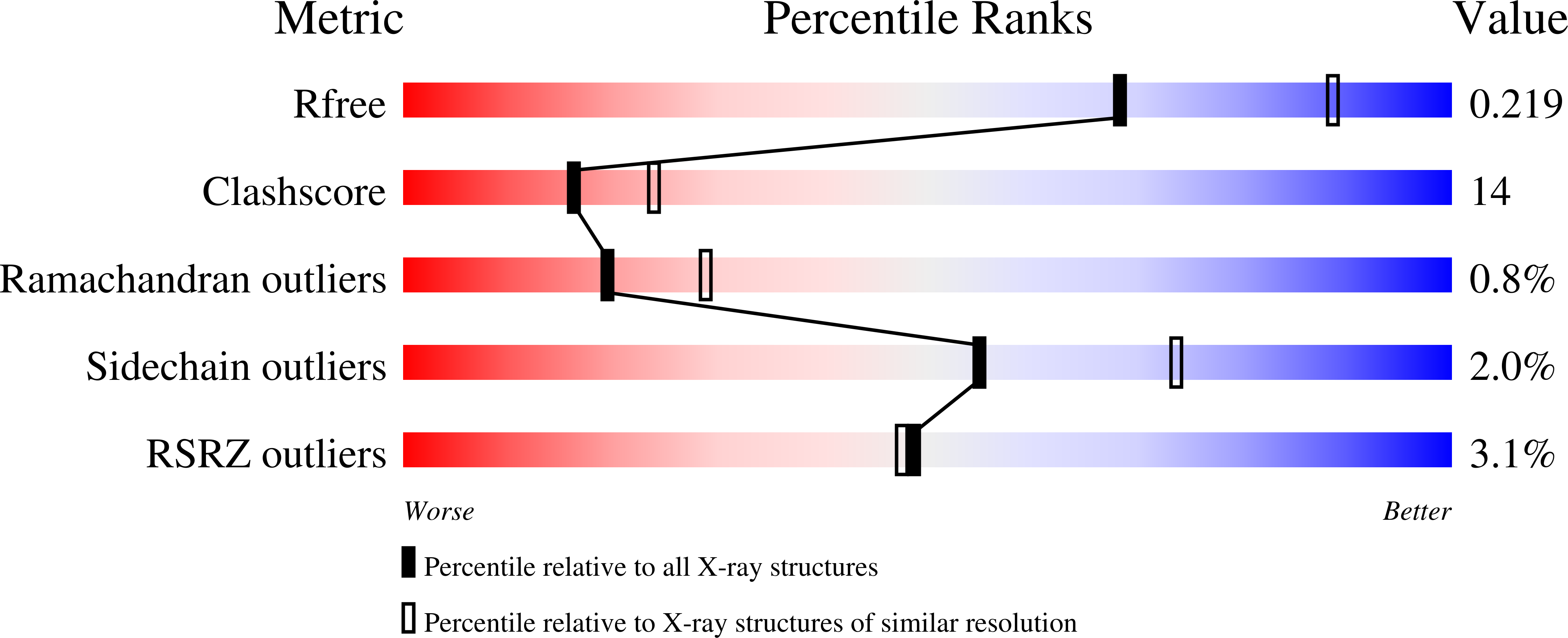
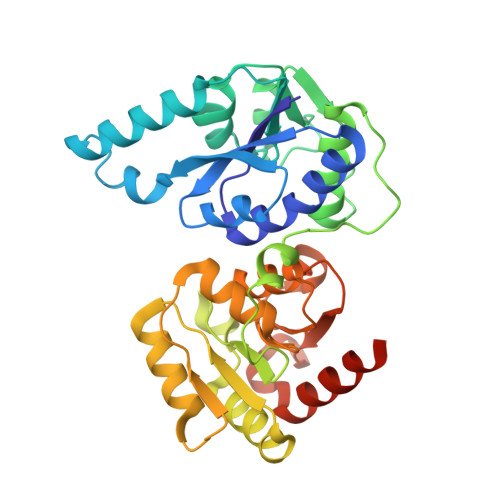
Structure of the Escherichia coli heptosyltransferase WaaC: binary complexes with ADP and ADP-2-deoxy-2-fluoro heptose.
Grizot, S., Salem, M., Vongsouthi, V., Durand, L., Moreau, F., Dohi, H., Vincent, S., Escaich, S., Ducruix, A.(2006) J Mol Biol 363: 383-394
- PubMed: 16963083
- DOI: https://doi.org/10.1016/j.jmb.2006.07.057
- Primary Citation of Related Structures:
2GT1, 2H1F, 2H1H - PubMed Abstract:
Lipopolysaccharides constitute the outer leaflet of the outer membrane of Gram-negative bacteria and are therefore essential for cell growth and viability. The heptosyltransferase WaaC is a glycosyltransferase (GT) involved in the synthesis of the inner core region of LPS. It catalyzes the addition of the first L-glycero-D-manno-heptose (heptose) molecule to one 3-deoxy-D-manno-oct-2-ulosonic acid (Kdo) residue of the Kdo2-lipid A molecule. Heptose is an essential component of the LPS core domain; its absence results in a truncated lipopolysaccharide associated with the deep-rough phenotype causing a greater susceptibility to antibiotic and an attenuated virulence for pathogenic Gram-negative bacteria. Thus, WaaC represents a promising target in antibacterial drug design. Here, we report the structure of WaaC from the Escherichia coli pathogenic strain RS218 alone at 1.9 A resolution, and in complex with either ADP or the non-cleavable analog ADP-2-deoxy-2-fluoro-heptose of the sugar donor at 2.4 A resolution. WaaC adopts the GT-B fold in two domains, characteristic of one glycosyltransferase structural superfamily. The comparison of the three different structures shows that WaaC does not undergo a domain rotation, characteristic of the GT-B family, upon substrate binding, but allows the substrate analog and the reaction product to adopt remarkably distinct conformations inside the active site. In addition, both binary complexes offer a close view of the donor subsite and, together with results from site-directed mutagenesis studies, provide evidence for a model of the catalytic mechanism.
Organizational Affiliation:
Laboratoire de Cristallographie et RMN Biologiques, UMR 8015 CNRS, Université Paris Descartes, Faculté de Pharmacie, 4, Avenue de l'Observatoire, F-75270 Paris cedex 06, France.