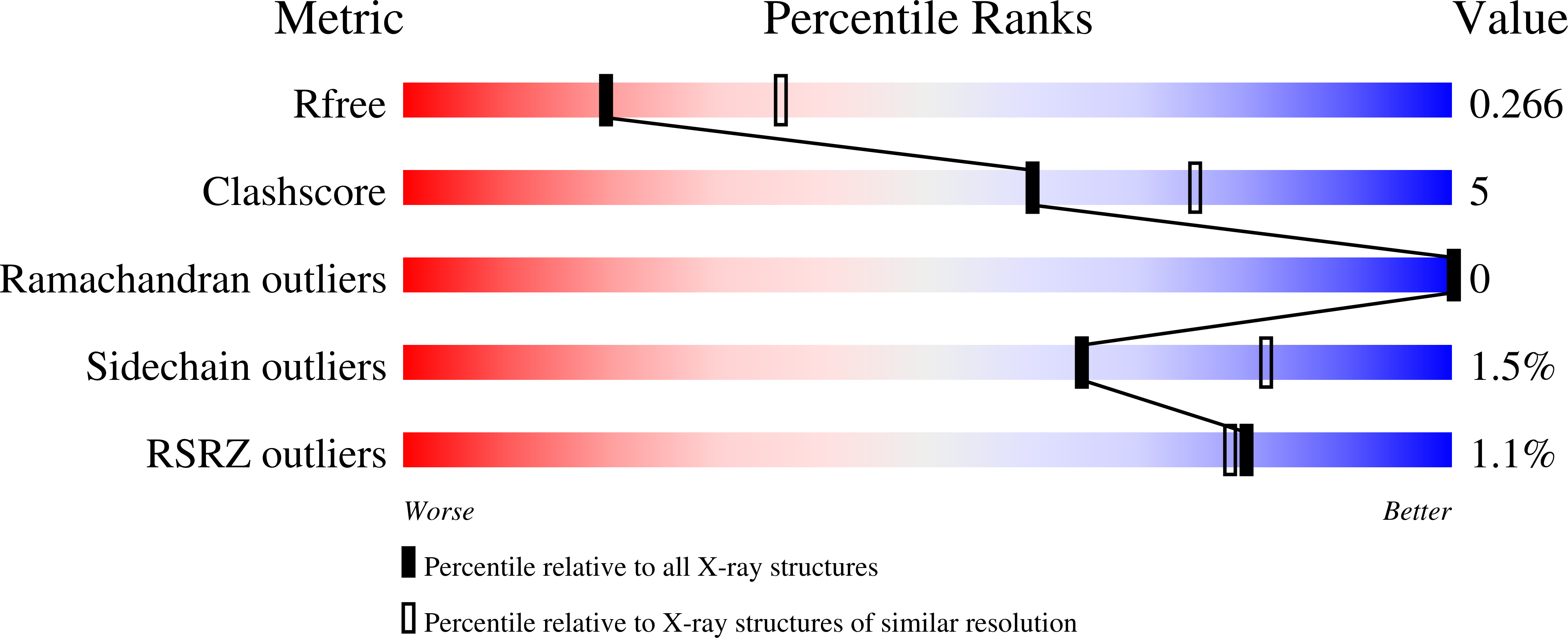
Crystal structure of Arabidopsis thaliana 12-oxophytodienoate reductase isoform 3 in complex with 8-iso prostaglandin A(1).
Han, B.W., Malone, T.E., Kim, D.J., Bingman, C.A., Kim, H.J., Fox, B.G., Phillips, G.N.(2011) Proteins 79: 3236-3241
- PubMed: 21915915
- DOI: https://doi.org/10.1002/prot.23153
- Primary Citation of Related Structures:
2G5W - PubMed Abstract:
12-Oxophytodienoate reductase 3 (OPR3), one of the enzymes involved in the biosynthesis of the plant hormone jasmonic acid (JA), catalyzes the reduction of the cyclopentenone ring of (9 S ,13 S )-12-oxophytodienoate [(9 S ,13 S )-OPDA]. However, there has been no structural information about the interaction between OPRs and the physiologically relevant (9 S ,13 S )-OPDA. Here we report the crystal structure of Arabidopsis thaliana OPR3 in complex with 8- iso prostaglandin A1 (8- iso PGA 1 ) which has the same stereochemistry in the cyclopentenone ring as in the physiologically relevant 9 S ,13 S -OPDA. This structure reveals a new binding mode for substrate that likely contributes to the relaxed stereospecificity observed for AtOPR3.
Organizational Affiliation:
Research Institute of Pharmaceutical Sciences, College of Pharmacy, Seoul National University, Seoul 151-742, Korea. bwhan@snu.ac.kr