Understanding GFP Posttranslational Chemistry: Structures of Designed Variants that Achieve Backbone Fragmentation, Hydrolysis, and Decarboxylation.
Barondeau, D.P., Kassmann, C.J., Tainer, J.A., Getzoff, E.D.(2006) J Am Chem Soc 128: 4685-4693
- PubMed: 16594705
- DOI: https://doi.org/10.1021/ja056635l
- Primary Citation of Related Structures:
2G16, 2G2S, 2G3D, 2G5Z, 2G6E - PubMed Abstract:
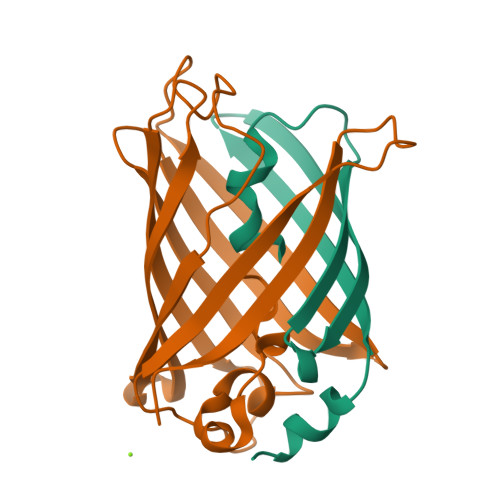
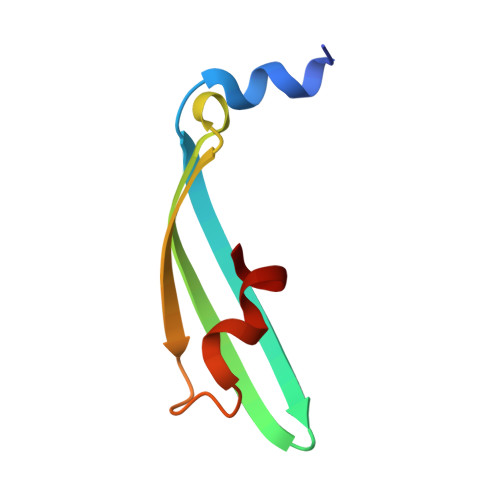
The green fluorescent protein (GFP) creates a fluorophore out of three sequential amino acids by promoting spontaneous posttranslational modifications. Here, we use high-resolution crystallography to characterize GFP variants that not only undergo peptide backbone cyclization but additional denaturation-induced peptide backbone fragmentation, native peptide hydrolysis, and decarboxylation reactions. Our analyses indicate that architectural features that favor GFP peptide cyclization also drive peptide hydrolysis. These results are relevant for the maturation pathways of GFP homologues, such as the kindling fluorescent protein and the Kaede protein, which use backbone cleavage to red-shift the spectral properties of their chromophores. We further propose a photochemical mechanism for the decarboxylation reaction, supporting a role for the GFP protein environment in facilitating radical formation and one-electron chemistry, which may be important in activating oxygen for the oxidation step of chromophore biosynthesis. Together, our results characterize GFP posttranslational modification chemistry with implications for the energetic landscape of backbone cyclization and subsequent reactions, and for the rational design of predetermined spontaneous backbone cyclization and cleavage reactions.
Organizational Affiliation:
Department of Molecular Biology, The Skaggs Institute for Chemical Biology, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, California 92037, USA.