Different poses for ligand and chaperone in inhibitor-bound Hsp90 and GRP94: implications for paralog-specific drug design.
Immormino, R.M., Metzger, L.E., Reardon, P.N., Dollins, D.E., Blagg, B.S., Gewirth, D.T.(2009) J Mol Biol 388: 1033-1042
- PubMed: 19361515
- DOI: https://doi.org/10.1016/j.jmb.2009.03.071
- Primary Citation of Related Structures:
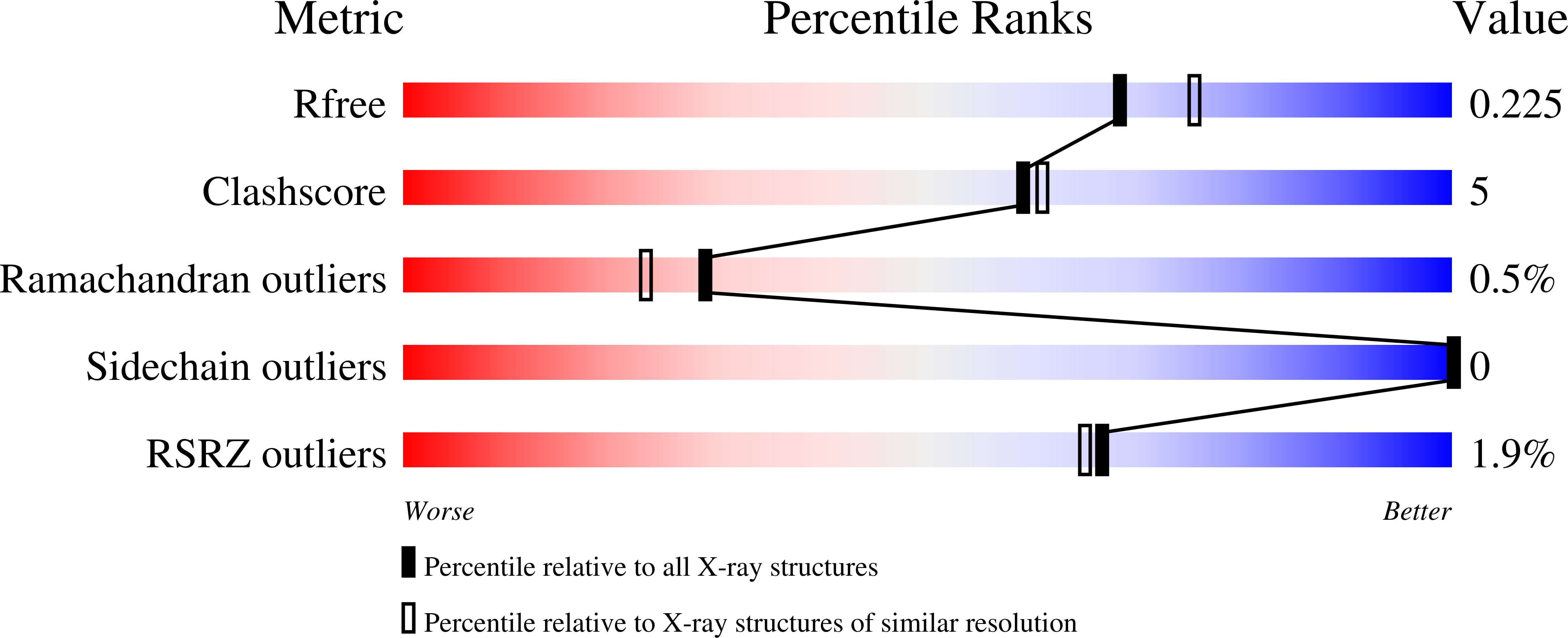
2EXL, 2FXS, 2GFD - PubMed Abstract:
Hsp90 chaperones contain an N-terminal ATP binding site that has been effectively targeted by competitive inhibitors. Despite the myriad of inhibitors, none to date have been designed to bind specifically to just one of the four mammalian Hsp90 paralogs, which are cytoplasmic Hsp90alpha and beta, endoplasmic reticulum GRP94, and mitochondrial Trap-1. Given that each of the Hsp90 paralogs is responsible for chaperoning a distinct set of client proteins, specific targeting of one Hsp90 paralog may result in higher efficacy and therapeutic control. Specific inhibitors may also help elucidate the biochemical roles of each Hsp90 paralog. Here, we present side-by-side comparisons of the structures of yeast Hsp90 and mammalian GRP94, bound to the pan-Hsp90 inhibitors geldanamycin (Gdm) and radamide. These structures reveal paralog-specific differences in the Hsp90 and GRP94 conformations in response to Gdm binding. We also report significant variation in the pose and disparate binding affinities for the Gdm-radicicol chimera radamide when bound to the two paralogs, which may be exploited in the design of paralog-specific inhibitors.
Organizational Affiliation:
Hauptman-Woodward Medical Research Institute, Buffalo, NY 14203, USA.