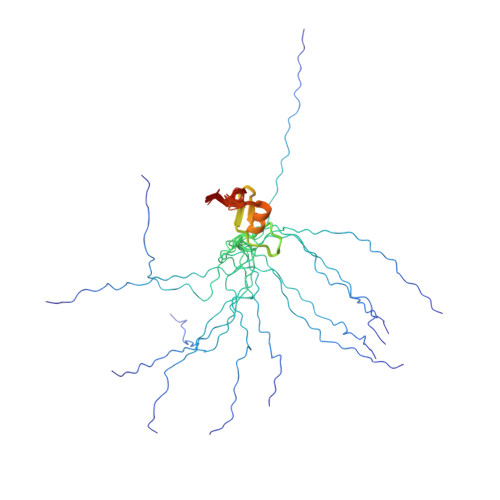
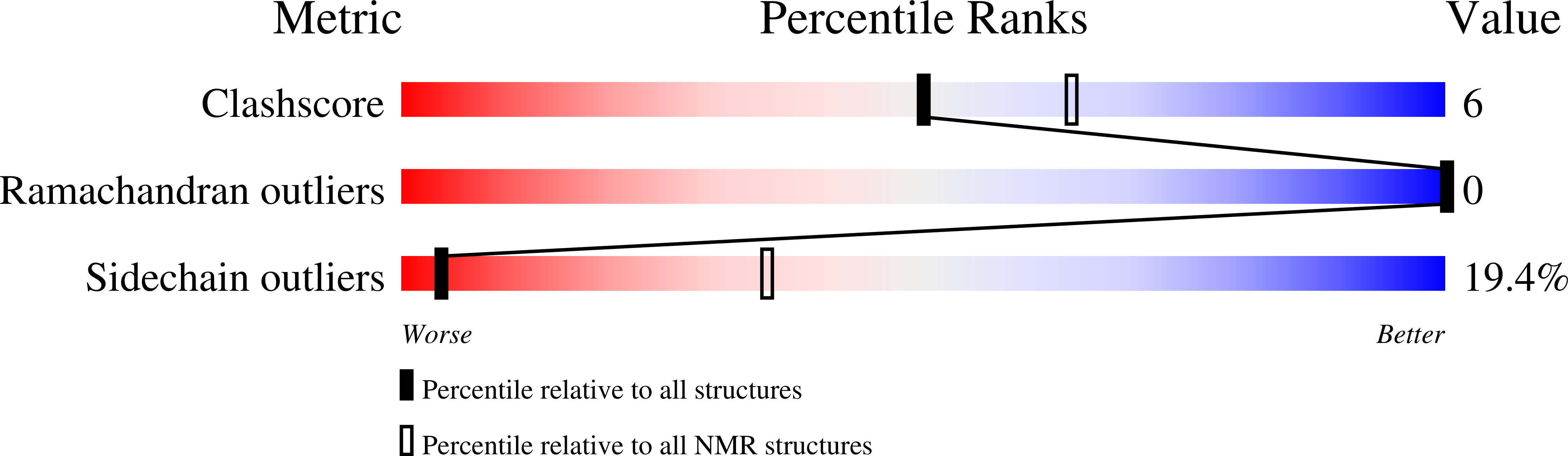Solution Structure of the RBCC/TRIM B-box1 Domain of Human MID1: B-box with a RING.
Massiah, M.A., Simmons, B.N., Short, K.M., Cox, T.C.(2006) J Mol Biology 358: 532-545
- PubMed: 16529770
- DOI: https://doi.org/10.1016/j.jmb.2006.02.009
- Primary Citation of Related Structures:
2FFW - PubMed Abstract:
B-box domains are a defining feature of the tripartite RBCC (RING, B-box, coiled-coil) or TRIM proteins, many of which are E3 ubiquitin ligases. However, little is known about the biological function of B-boxes. In some RBCC/TRIM proteins there is only a single B-box (type 2) domain, while others have both type 1 and type 2 B-box domains in tandem adjacent to their RING domain. These two types of B-boxes share little sequence similarity, except the presence of cysteine and histidine residues: eight in most B-box1 domains and seven in B-box2 domains. We report here the high-resolution solution structure of the first B-box1 domain (from the human RBCC protein, MID1) based on 670 nuclear Overhauser effect (NOE)-derived distance restraints, 12 hydrogen bonds, and 44 dihedral angles. The domain consists of a three-turn alpha-helix, two short beta-strands, and three beta-turns, encompassing Val117 to Pro164, which binds two zinc atoms. One zinc atom is coordinated by cysteine residues 119, 122, 142, 145, while cysteine 134, 137 and histidine 150, 159 coordinate the other. This topology is markedly different from the only other B-box structure reported; that of a type 2 B-box from Xenopus XNF7, which binds a single zinc atom. Of note, the B-box1 structure closely resembles the folds of the RING, ZZ and U-box domains of E3 and E4 ubiquitin enzymes, raising the possibility that the B-box1 domain either has E3 activity itself or enhances the activity of RING type E3 ligases (i.e. confers E4 enzyme activity). The structure of the MID1 B-box1 also reveals two potential protein interaction surfaces. One of these is likely to provide the binding interface for Alpha 4 that is required for the localized turnover of the catalytic subunit of PP2A, the major Ser/Thr phosphatase.
Organizational Affiliation:
Department of Biochemistry and Molecular Biology, Oklahoma State University, Stillwater, OK 74075 USA. massiah@biochem.okstate.edu