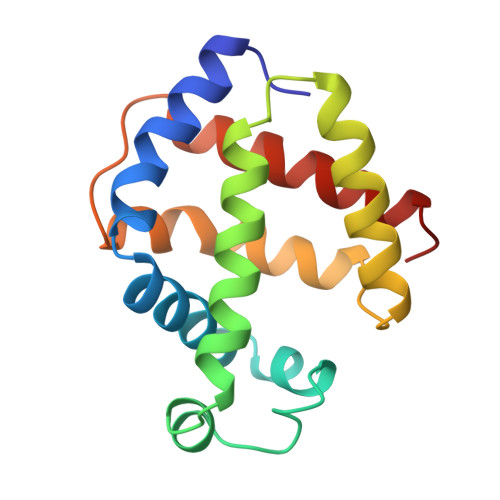
X-ray crystal structure of ferric Aplysia limacina myoglobin in different liganded states.
Conti, E., Moser, C., Rizzi, M., Mattevi, A., Lionetti, C., Coda, A., Ascenzi, P., Brunori, M., Bolognesi, M.(1993) J Mol Biol 233: 498-508
- PubMed: 8411158
- DOI: https://doi.org/10.1006/jmbi.1993.1527
- Primary Citation of Related Structures:
2FAL, 2FAM - PubMed Abstract:
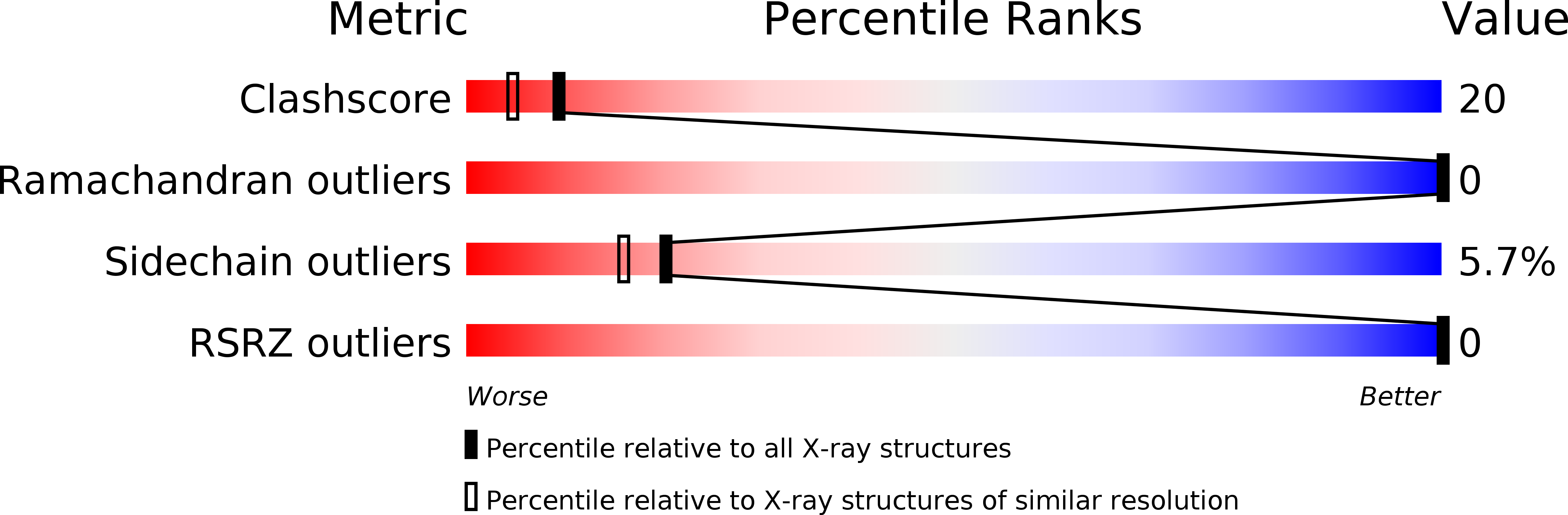
The X-ray crystal structure of the ligand-free ferric form of Aplysia limacina myoglobin (pH 6.0) has been refined at 1.7 A resolution (R = 15.1%), and its cyanide, thiocyanate and imidazole derivatives studied by difference Fourier techniques at atomic resolution. The crystallographic R-factors of the three different derivatives reported are 16.1%, 16.1% and 15.6% at 1.8 A, 2.0 A and 2.0 A resolution, respectively. The present results have been analyzed in parallel with previous crystallographic studies on the molecular structures of the fluoride and azide derivatives of ferric Aplysia limacina myoglobin. Ligand binding to the distal site of the heme pocket results in different networks of hydrogen bonds involving to various degrees the bound ligand, residue Arg(66)E10, the heme propionate III, ordered water molecules and/or protein backbone atoms from the CD region. In particular, Arg(66)E10 stabilizes the bound ligand and compensates for the absence of the hydrogen bond donor residue HisE7, commonly present in oxygen-carrying globins.
Organizational Affiliation:
Dipartimento di Genetica e Microbiologia, Università di Pavia, Italy.