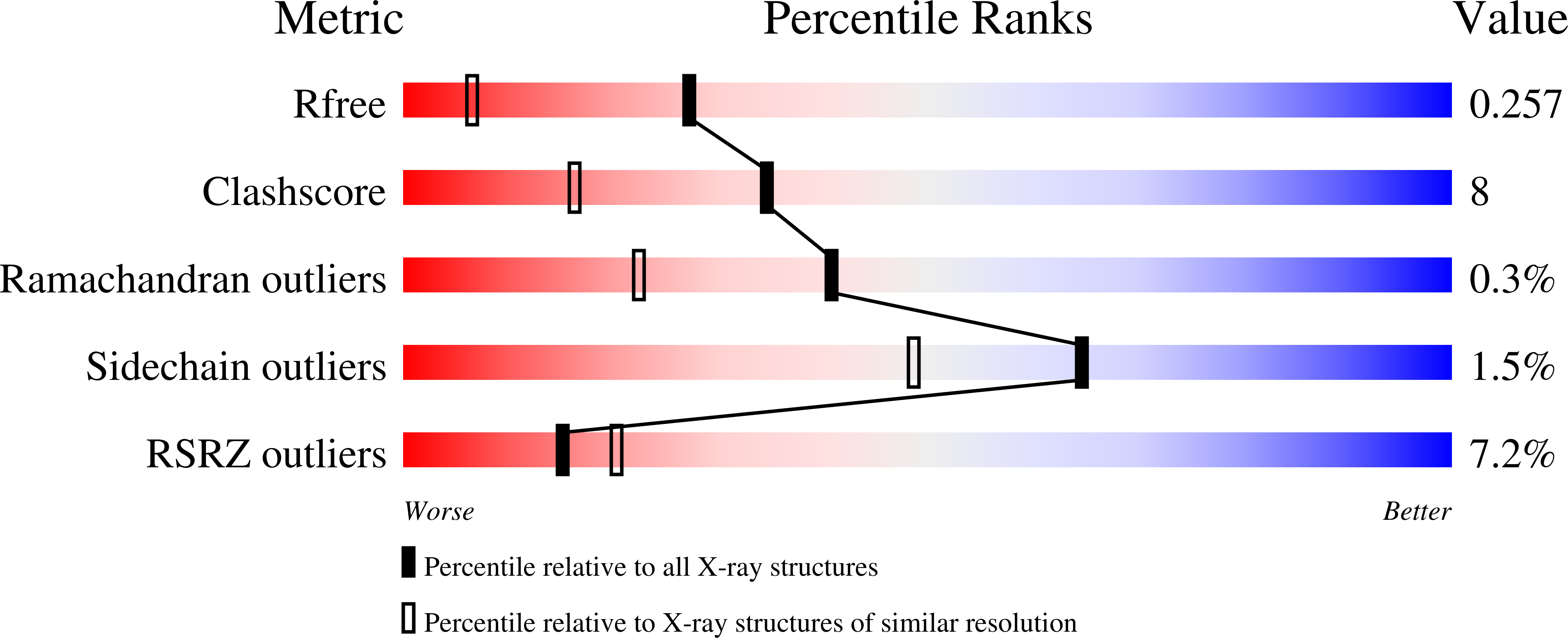
Structure of a novel N-acetyl-L-citrulline deacetylase from Xanthomonas campestris
Shi, D., Yu, X., Roth, L., Tuchman, M., Allewell, N.M.(2007) Biophys Chem 126: 86-93
- PubMed: 16750290
- DOI: https://doi.org/10.1016/j.bpc.2006.05.013
- Primary Citation of Related Structures:
2F7V, 2F8H - PubMed Abstract:
The structure of a novel acetylcitrulline deacetylase from the plant pathogen Xanthomonas campestris has been solved by multiple-wavelength anomalous dispersion (MAD) using crystals grown from selenomethionine-substituted protein and refined at 1.75 A resolution. The asymmetric unit of the crystal contains one monomer consisting of two domains, a catalytic domain and a dimerization domain. The catalytic domain is able to bind a single Co(II) ion at the active site with no change in conformation. The dimerization domain forms an interface between two monomers related by a crystallographic two-fold symmetry axis. The interface is maintained by hydrophobic interactions between helices and hydrogen bonding between two beta strands that form a continuous beta sheet across the dimer interface. Because the dimers are also related by two-fold crystallographic axes, they pack together across the crystal via the dimerization domain, suggesting that higher order oligomers may form in solution. The polypeptide fold of the monomer is similar to the fold of Pseudomonas sp. carboxypeptidase G2 and Neisseria meningitidis succinyl diaminopimelate desuccinylase. Structural comparison among these enzymes allowed modeling of substrate binding and suggests a possible catalytic mechanism, in which Glu130 functions as a bifunctional general acid-base catalyst and the metal ion polarizes the carbonyl of the acetyl group.
Organizational Affiliation:
Children's Research Institute, Children's National Medical Center, 111 Michigan Avenue, N.W., Washington, DC 20010-2970, USA. dshi@cnmcresearch.org