Structure and ligand binding properties of myoglobins reconstituted with monodepropionated heme: functional role of each heme propionate side chain
Harada, K., Makino, M., Sugimoto, H., Hirota, S., Matsuo, T., Shiro, Y., Hisaeda, Y., Hayashi, T.(2007) Biochemistry 46: 9406-9416
- PubMed: 17636874
- DOI: https://doi.org/10.1021/bi7007068
- Primary Citation of Related Structures:
2EKT, 2EKU - PubMed Abstract:
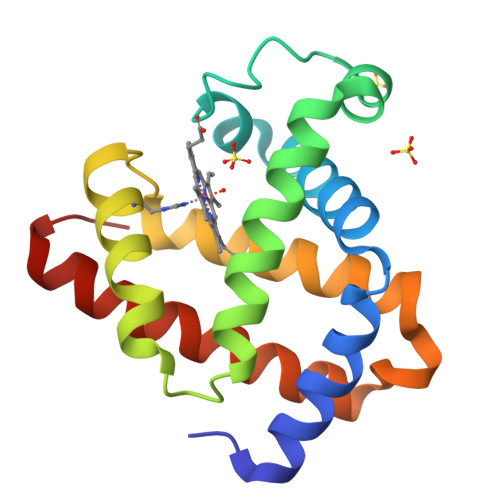
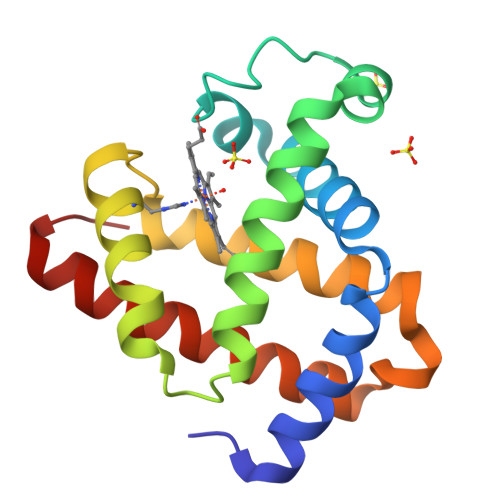
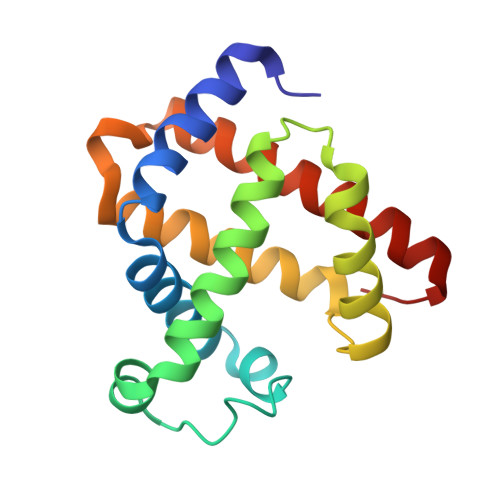
Two heme propionate side chains, which are attached at the 6 and 7 positions of the heme framework, are linked with Arg45 and Ser92, respectively, in sperm whale myoglobin. To evaluate the role of each propionate, two kinds of one-legged hemins, 6-depropionated and 7-depropionated protohemins, were prepared and inserted into the apomyoglobin to yield two reconstituted proteins. Structural data of the reconstituted myoglobins were obtained via an X-ray crystallographic analysis at a resolution of 1.1-1.4 A and resonance Raman spectroscopy. It was found that the lack of the 6-propionate reduces the number of hydrogen bonds in the distal site and clearly changes the position of the Arg45 residue with the disrupting Arg45-Asp60 interaction. In contrast, the removal of the 7-propionate does not cause a significant structural change in the residues of the distal and proximal sites. However, the resonance Raman studies suggested that the coordination bond strength of the His93-Fe bond for the protein with the 7-depropionated protoheme slightly increases compared to that for the protein with the native heme. The O2 and CO ligand binding studies for the reconstituted proteins with the one-legged hemes provide an important insight into the functional role of each propionate. The lack of the 6-propionate accelerates the O2 dissociation by ca. 3-fold compared to those of the other reconstituted and native proteins. The lack of the 7-propionate enhances the CO affinity by 2-fold compared to that of the protein with the native heme. These results indicate that the 6-propionate clearly contributes to the stabilization of the bound O2, whereas the 7-propionate plays an important role in the regulation of the Fe-His bond.
Organizational Affiliation:
Department of Applied Chemistry, Graduate School of Engineering, Osaka University, Suita 565-0871, Japan.