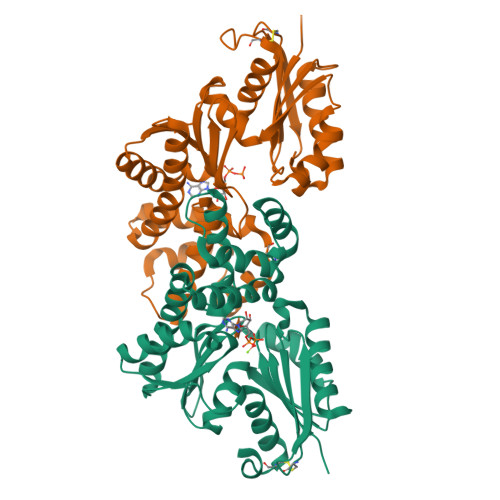
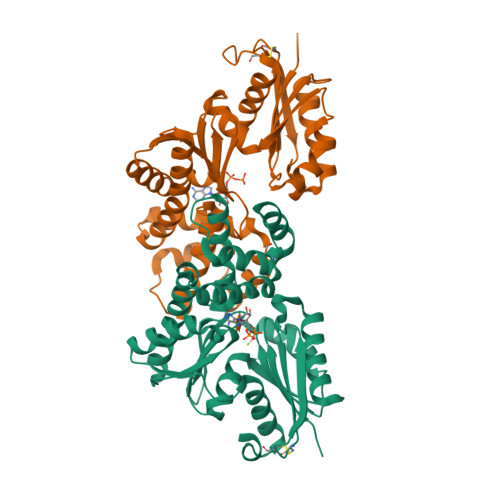
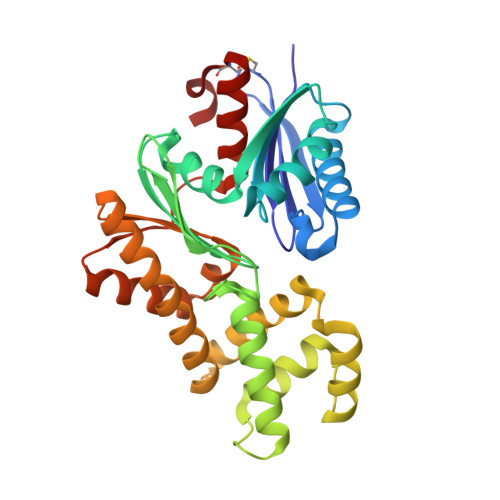
Crystal structures of an ATP-dependent hexokinase with broad substrate specificity from the hyperthermophilic archaeon Sulfolobus tokodaii.
Nishimasu, H., Fushinobu, S., Shoun, H., Wakagi, T.(2007) J Biological Chem 282: 9923-9931
- PubMed: 17229727
- DOI: https://doi.org/10.1074/jbc.M610678200
- Primary Citation of Related Structures:
2E2N, 2E2O, 2E2P, 2E2Q - PubMed Abstract:
Hexokinase catalyzes the phosphorylation of glucose to glucose 6-phosphate by using ATP as a phosphoryl donor. Recently, we identified and characterized an ATP-dependent hexokinase (StHK) from the hyperthermophilic archaeon Sulfolobus tokodaii, which can phosphorylate a broad range of sugar substrates, including glucose, mannose, glucosamine, and N-acetylglucosamine. Here we present the crystal structures of StHK in four different forms: (i) apo-form, (ii) binary complex with glucose, (iii) binary complex with ADP, and (iv) quaternary complex with xylose, Mg(2+), and ADP. Forms i and iii are in the open state, and forms ii and iv are in the closed state, indicating that sugar binding induces a large conformational change, whereas ADP binding does not. The four different crystal structures of the same enzyme provide "snapshots" of the conformational changes during the catalytic cycle. StHK exhibits a core fold characteristic of the hexokinase family, but the structures of several loop regions responsible for substrate binding are significantly different from those of other known hexokinase family members. Structural comparison of StHK with human N-acetylglucosamine kinase and other hexokinases provides an explanation for the ability of StHK to phosphorylate both glucose and N-acetylglucosamine. A Mg(2+) ion and coordinating water molecules are well defined in the electron density of the quaternary complex structure. This structure represents the first direct visualization of the binding mode for magnesium to hexokinase and thus allows for a better understanding of the catalytic mechanism proposed for the entire hexokinase family.
Organizational Affiliation:
Department of Biotechnology, the University of Tokyo, 1-1-1 Yayoi, Bunkyo-ku, Tokyo 113-8657, Japan.