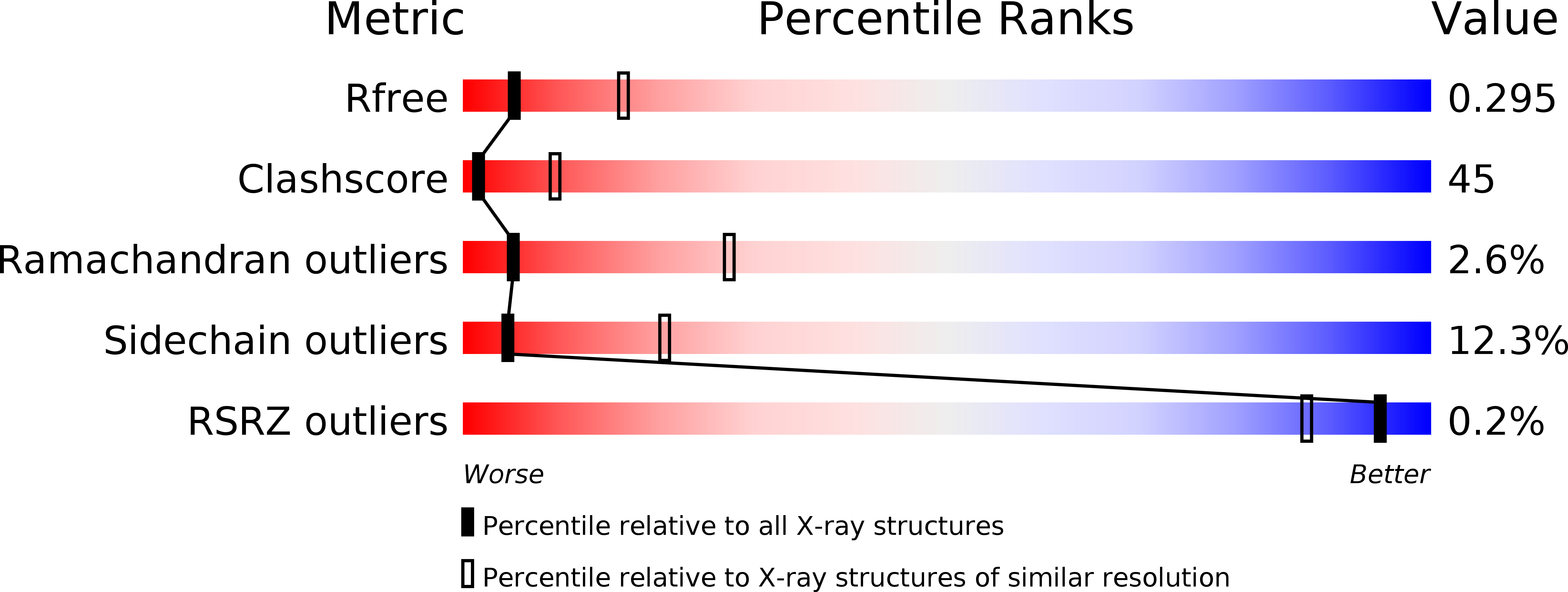
Release of a damaged cofactor from a coenzyme B12-dependent enzyme: X-ray structures of diol dehydratase-reactivating factor
Shibata, N., Mori, K., Hieda, N., Higuchi, Y., Yamanishi, M., Toraya, T.(2005) Structure 13: 1745-1754
- PubMed: 16338403
- DOI: https://doi.org/10.1016/j.str.2005.08.011
- Primary Citation of Related Structures:
2D0O, 2D0P - PubMed Abstract:
The crystal structures of ADP bound and nucleotide-free forms of molecular chaperone-like diol dehydratase-reactivating factor (DDR) were determined at 2.0 and 3.0 A, respectively. DDR exists as a dimer of heterodimer (alphabeta)2. The alpha subunit has four domains: ATPase domain, swiveling domain, linker domain, and insert domain. The beta subunit, composed of a single domain, has a similar fold to the beta subunit of diol dehydratase (DD). The binding of an ADP molecule to the nucleotide binding site of DDR causes a marked conformational change of the ATPase domain of the alpha subunit, which would weaken the interactions between the DDR alpha and beta subunits and make the displacement of the DDR beta subunit by DD through the beta subunit possible. The binding of the DD beta subunit to the DDR alpha subunit induces steric repulsion between the DDR alpha and DD alpha subunits that would lead to the release of a damaged cofactor from inactivated holoDD.
Organizational Affiliation:
Graduate School of Life Science, University of Hyogo, 3-2-1 Kouto, Ako-gun, Hyogo 678-1297, Japan.