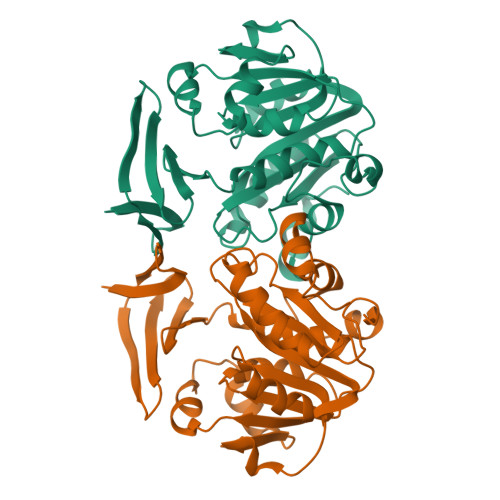
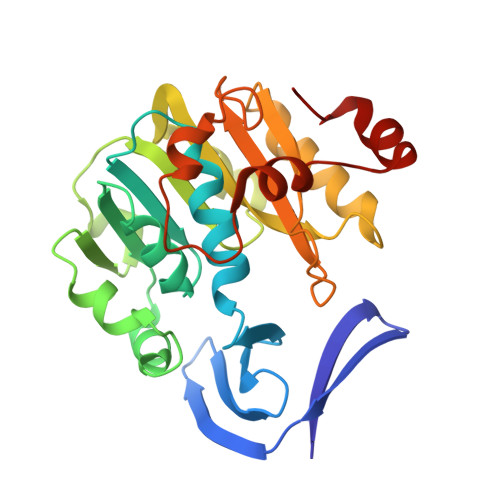
Crystal Structure of Helicobacter Pylori Spermidine Synthase: A Rossmann-Like Fold with a Distinct Active Site
Lu, P.-K., Tsai, J.-Y., Chien, H.Y., Huang, H., Chu, C.-H., Sun, Y.-J.(2007) Proteins 67: 743
- PubMed: 17357156
- DOI: https://doi.org/10.1002/prot.21315
- Primary Citation of Related Structures:
2CMG, 2CMH - PubMed Abstract:
Spermidine synthase (putrescine aminopropyltransferase, PAPT) catalyzes the transfer of the aminopropyl group from decarboxylated S-adenosylmethionine to putrescine during spermidine biosynthesis. Helicobacter pylori PAPT (HpPAPT) has a low sequence identity with other PAPTs and lacks the signature sequence found in other PAPTs. The crystal structure of HpPAPT, determined by multiwavelength anomalous dispersion, revealed an N-terminal beta-stranded domain and a C-terminal Rossmann-like domain. Structural comparison with other PAPTs showed that HpPAPT has a unique binding pocket between two domains, numerous non-conserved residues, a less acidic electrostatic surface potential, and a large buried space within the structure. HpPAPT lacks the gatekeeping loop that facilitates substrate binding in other PAPTs. PAPTs are essential for bacterial cell viability; thus, HpPAPT may be a potential antimicrobial drug target for H. pylori owing to its characteristic PAPT sequence and distinct conformation.
Organizational Affiliation:
Institute of Bioinformatics and Structural Biology, National Tsing Hua University, Hsinchu 300, Taiwan, Republic of China.