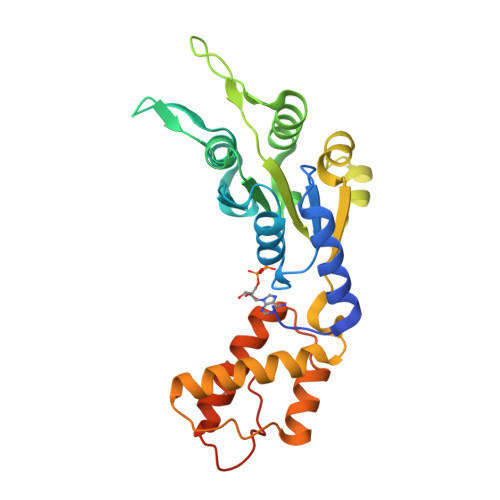
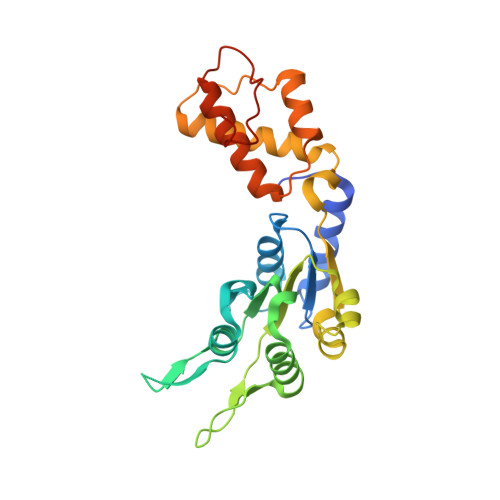
Structural Basis of the Nucleotide Driven Conformational Changes in the Aaa(+) Domain of Transcription Activator Pspf.
Rappas, M., Schumacher, J., Niwa, H., Buck, M., Zhang, X.(2006) J Mol Biology 357: 481
- PubMed: 16430918
- DOI: https://doi.org/10.1016/j.jmb.2005.12.052
- Primary Citation of Related Structures:
2C96, 2C98, 2C99, 2C9C - PubMed Abstract:
Bacterial enhancer-binding proteins (EBP) activate transcription by hydrolyzing ATP to restructure the sigma(54)-RNA polymerase-promoter complex. We compare six high resolution structures (<2.1 A) of the AAA(+) domain of EBP phage shock protein F (PspF) including apo, AMPPNP, Mg(2+)-ATP, and ADP forms. These structures permit a description of the atomic details underpinning the origins of the conformational changes occurring during ATP hydrolysis. Conserved regions of PspF's AAA(+) domain respond distinctively to nucleotide binding and hydrolysis, suggesting functional roles during the hydrolysis cycle, which completely agree with those derived from activities of PspF mutated at these positions. We propose a putative atomic switch that is responsible for coupling structural changes in the nucleotide-binding site to the repositioning of the sigma(54)-interacting loops. Striking similarities in nucleotide-specific conformational changes and atomic switch exist between PspF and the large T antigen helicase, suggesting conservation in the origin of those events amongst AAA(+) proteins.
Organizational Affiliation:
Division of Molecular Biosciences, Imperial College London, London SW7 2AZ, UK.