Structural and Functional Insights Into the Interaction of Echoviruses and Decay-Accelerating Factor.
Pettigrew, D.M., Williams, D.T., Kerrigan, D., Evans, D.J., Lea, S.M., Bhella, D.(2006) J Biological Chem 281: 5169
- PubMed: 16272562
- DOI: https://doi.org/10.1074/jbc.M510362200
- Primary Citation of Related Structures:
2C8I - PubMed Abstract:
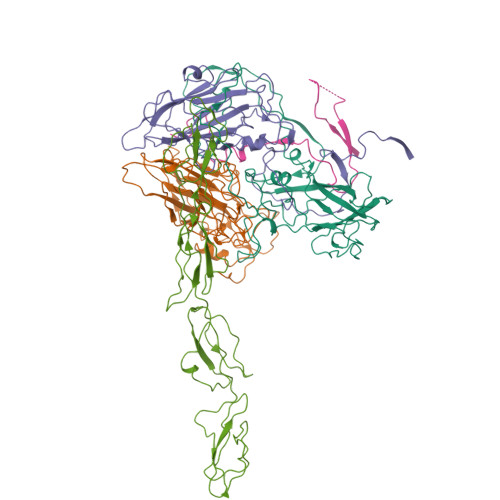
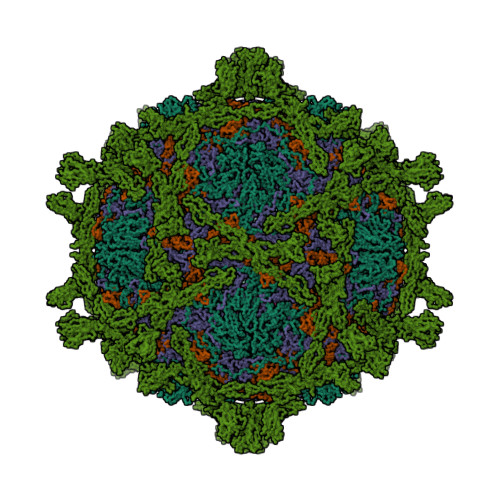
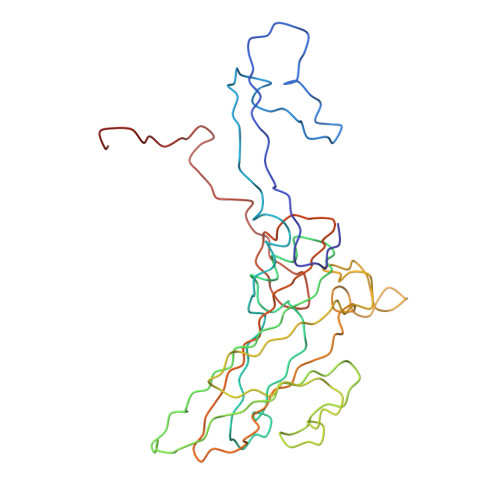
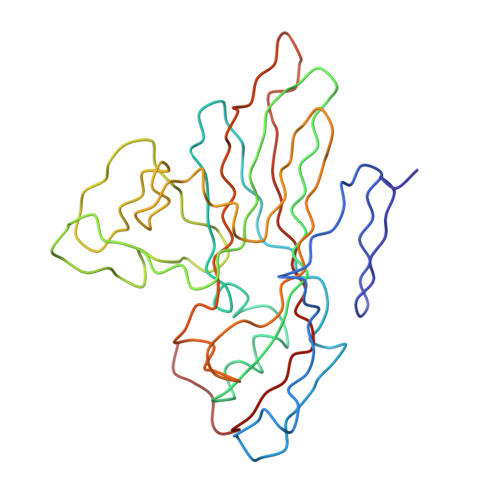
Many enteroviruses bind to the complement control protein decay-accelerating factor (DAF) to facilitate cell entry. We present here a structure for echovirus (EV) type 12 bound to DAF using cryo-negative stain transmission electron microscopy and three-dimensional image reconstruction to 16-A resolution, which we interpreted using the atomic structures of EV11 and DAF. DAF binds to a hypervariable region of the capsid close to the 2-fold symmetry axes in an interaction that involves mostly the short consensus repeat 3 domain of DAF and the capsid protein VP2. A bulge in the density for the short consensus repeat 3 domain suggests that a loop at residues 174-180 rearranges to prevent steric collision between closely packed molecules at the 2-fold symmetry axes. Detailed analysis of receptor interactions between a variety of echoviruses and DAF using surface plasmon resonance and comparison of this structure (and our previous work; Bhella, D., Goodfellow, I. G., Roversi, P., Pettigrew, D., Chaudhry, Y., Evans, D. J., and Lea, S. M. (2004) J. Biol. Chem. 279, 8325-8332) with reconstructions published for EV7 bound to DAF support major differences in receptor recognition among these viruses. However, comparison of the electron density for the two virus.receptor complexes (rather than comparisons of the pseudo-atomic models derived from fitting the coordinates into these densities) suggests that the dramatic differences in interaction affinities/specificities may arise from relatively subtle structural differences rather than from large-scale repositioning of the receptor with respect to the virus surface.
Organizational Affiliation:
Medical Research Council Virology Unit, Institute of Biomedical and Life Sciences, University of Glasgow, Scotland, United Kingdom.