The Crystal Structure of the Dps2 from Deinococcus Radiodurans Reveals an Unusual Pore Profile with a Non-Specific Metal Binding Site.
Cuypers, M.G., Mitchell, E.P., Romao, C.V., Mcsweeney, S.M.(2007) J Mol Biology 371: 787
- PubMed: 17583727
- DOI: https://doi.org/10.1016/j.jmb.2006.11.032
- Primary Citation of Related Structures:
2C2J, 2C6R - PubMed Abstract:
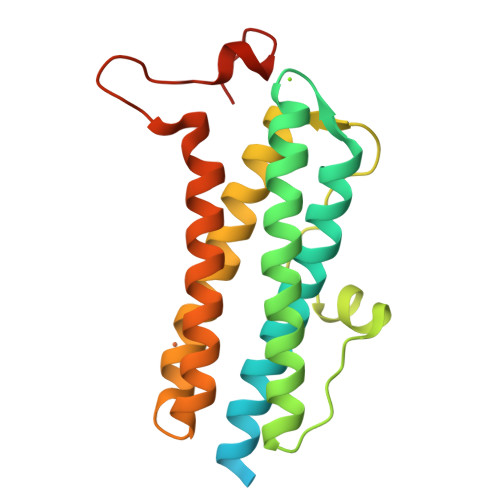
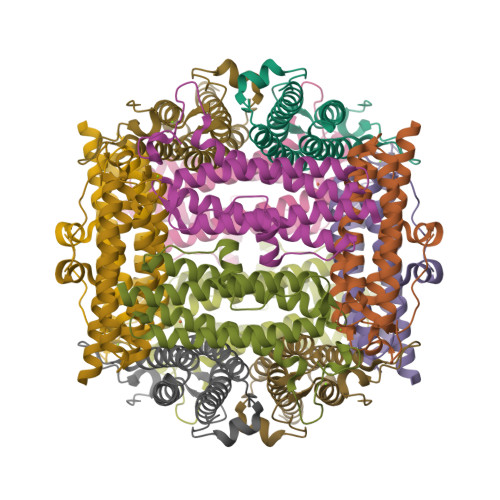
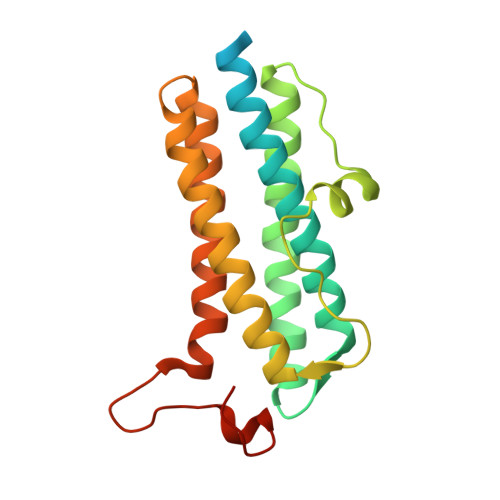
The crystal structure of recombinant Dps2 (DRB0092, DNA protecting protein under starved conditions) from the Gram-positive, radiation-resistant bacterium Deinococcus radiodurans has been determined in its apo and iron loaded states. Like other members of the Dps family, the bacterial DrDps2 assembles as a spherical dodecamer with an outer shell diameter of 90 A and an interior diameter of 40 A. A total of five iron sites were located in the iron loaded structure, representing the first stages of iron biomineralisation. Each subunit contains a mononuclear iron ferroxidase centre coordinated by residues highly conserved amongst the Dps family of proteins. In the structures presented, a distinct iron site is observed 6.1 A from the ferroxidase centre with a unique ligand configuration of mono coordination by the protein and no bridging ligand to the ferroxidase centre. A non-specific metallic binding site, suspected to play a regulative role in iron uptake/release from the cage, was found in a pocket located near to the external edge of the C-terminal 3-fold channel.
Organizational Affiliation:
ESRF (European Synchrotron Radiation Facility), 6 rue Jules Horowitz, BP 220, 38043 Grenoble, France.