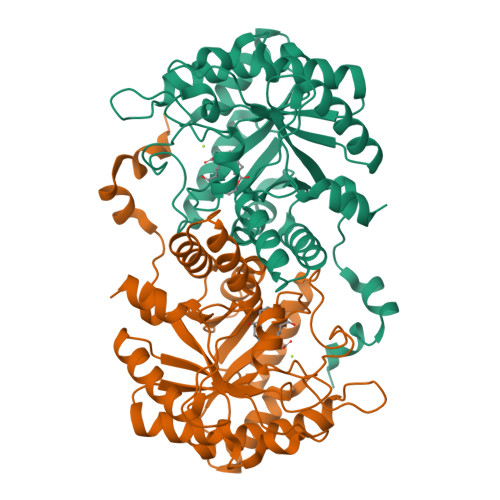
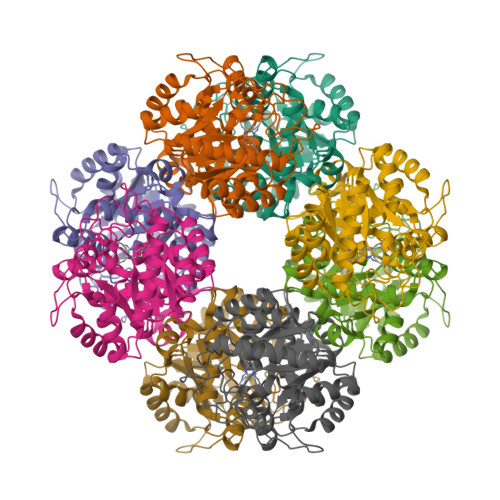
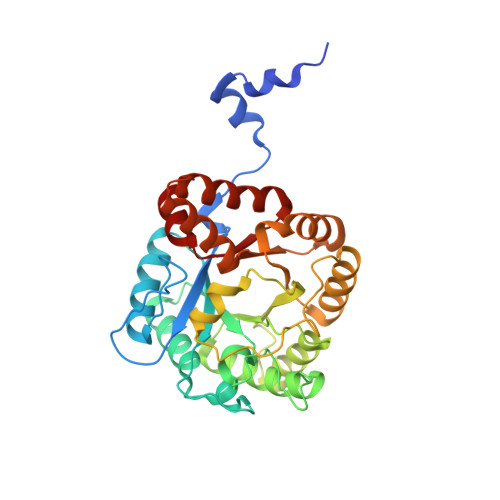
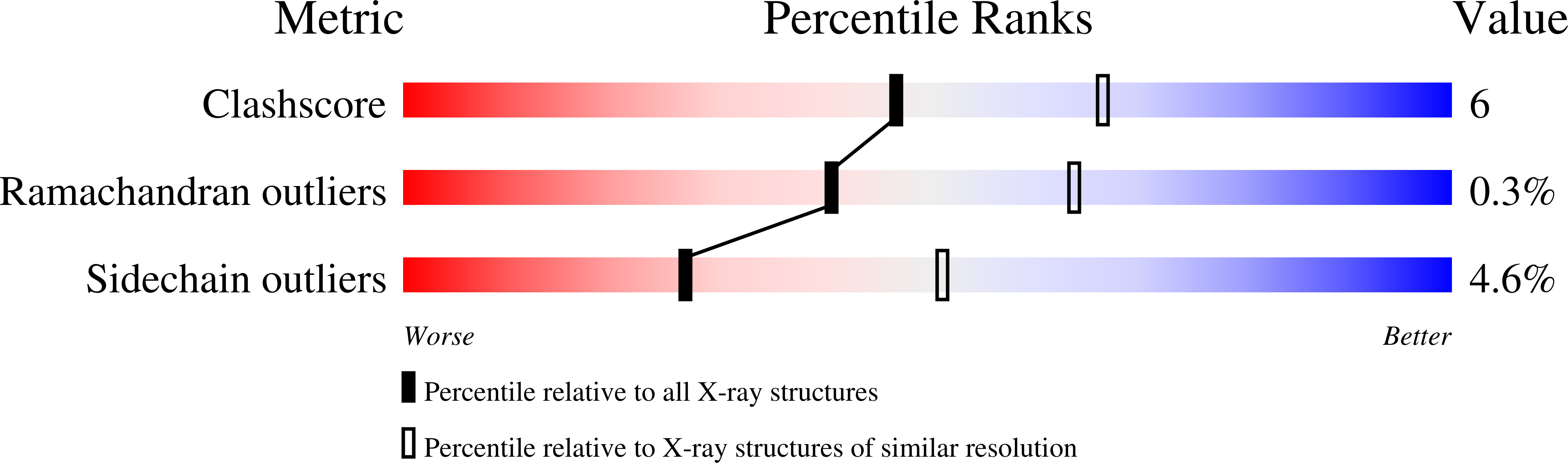Structure of Chlorobium Vibrioforme 5-Aminolaevulinic Acid Dehydratase Complexed with a Diacid Inhibitor.
Coates, L., Beaven, G., Erskine, P.T., Beale, S., Wood, S.P., Shoolingin-Jordan, P.M., Cooper, J.B.(2005) Acta Crystallogr D Biol Crystallogr 61: 1594
- PubMed: 16304458
- DOI: https://doi.org/10.1107/S0907444905030350
- Primary Citation of Related Structures:
2C1H - PubMed Abstract:
The structure of Chlorobium vibrioforme 5-aminolaevulinic acid dehydratase (ALAD) complexed with the irreversible inhibitor 4,7-dioxosebacic acid has been solved. The inhibitor binds by forming Schiff-base linkages with lysines 200 and 253 at the active site. The structure reported here provides a definition of the interactions made by both of the substrate molecules (A-side and P-side substrates) with the C. vibrioforme ALAD and is compared and contrasted with structures of the same inhibitor bound to Escherichia coli and yeast ALAD. The structure suggests why 4,7-dioxosebacic acid is a better inhibitor of the zinc-dependent ALADs than of the zinc-independent ALADs.
Organizational Affiliation:
Bioscience Division, Los Alamos National Laboratory, NM 87545, USA. lcoates@lanl.gov