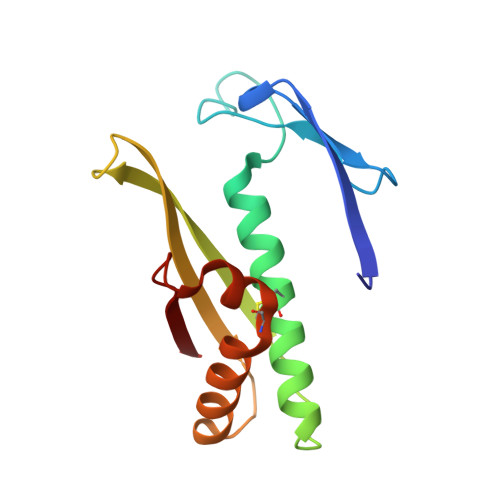
Structure of the Bacillus Subtilis Ohrb Hydroperoxide-Resistance Protein in a Fully Oxidized State.
Cooper, D.R., Surendranath, Y., Devedjiev, Y., Bielnicki, J., Derewenda, Z.S.(2007) Acta Crystallogr D Biol Crystallogr 63: 1269
- PubMed: 18084074
- DOI: https://doi.org/10.1107/S0907444907050226
- Primary Citation of Related Structures:
2BJO - PubMed Abstract:
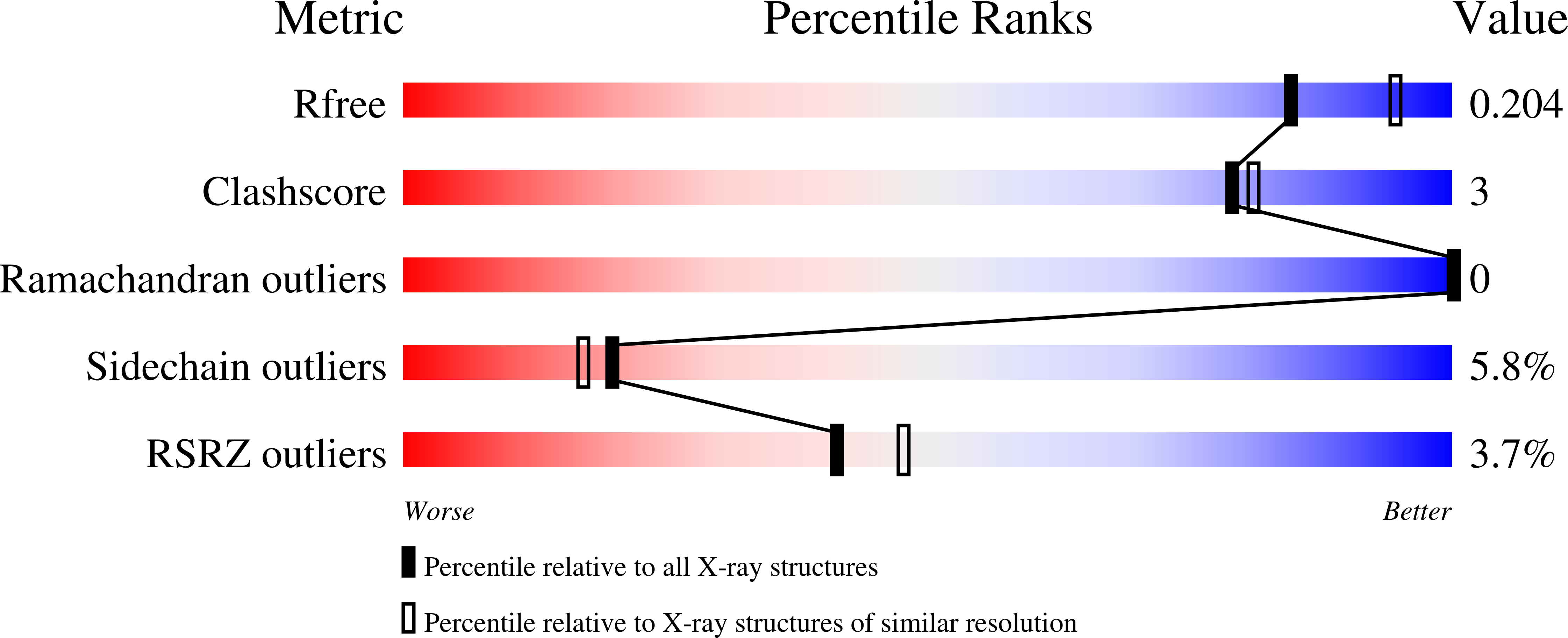
The crystal structure of the fully oxidized form of the Bacillus subtilis organic hydroperoxide-resistance (OhrB) protein is reported at 2.1 A resolution. The electron density reveals an intact catalytic disulfide bond (Cys55-Cys119) in each of the two molecules, which are intertwined into a canonical obligate dimer. However, the stereochemistry of the disulfides is unorthodox and strained, suggesting that they are sensitive to reducing agents. A deep solvent-accessible gorge reaching Cys55 may represent the access route for the reductant.
Organizational Affiliation:
Department of Molecular Physiology and Biological Physics, University of Virginia and the PSI2 Integrated Center for Structure-Function Innovation Charlottesville, Virginia 22908, USA.