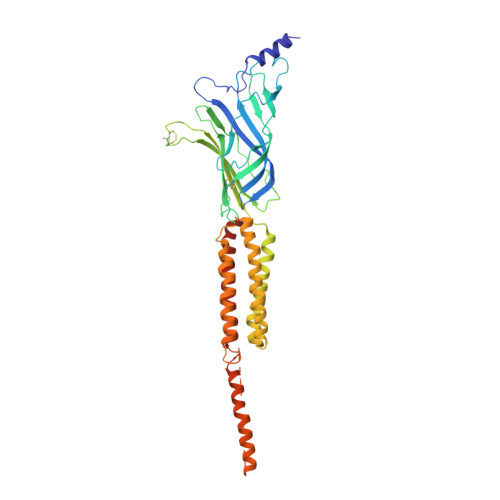
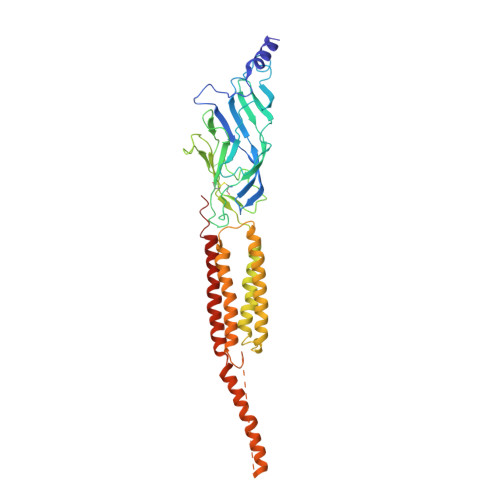
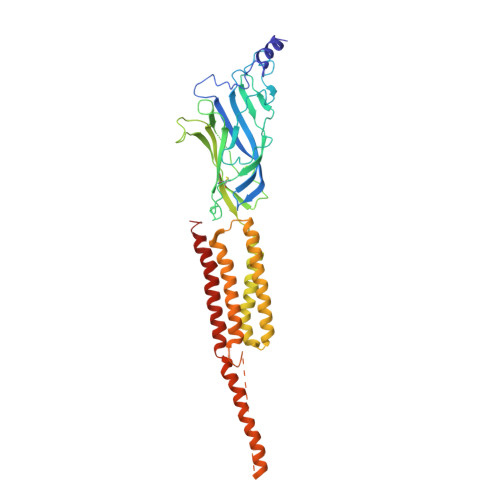
Refined Structure of the Nicotinic Acetylcholine Receptor at 4A Resolution
Unwin, N.(2005) J Mol Biology 346: 967
- PubMed: 15701510
- DOI: https://doi.org/10.1016/j.jmb.2004.12.031
- Primary Citation of Related Structures:
2BG9 - PubMed Abstract:
We present a refined model of the membrane-associated Torpedo acetylcholine (ACh) receptor at 4A resolution. An improved experimental density map was obtained from 342 electron images of helical tubes, and the refined structure was derived to an R-factor of 36.7% (R(free) 37.9%) by standard crystallographic methods, after placing the densities corresponding to a single molecule into an artificial unit cell. The agreement between experimental and calculated phases along the helical layer-lines was used to monitor progress in the refinement and to give an independent measure of the accuracy. The atomic model allowed a detailed description of the whole receptor in the closed-channel form, including the ligand-binding and intracellular domains, which have not previously been interpreted at a chemical level. We confirm that the two ligand-binding alpha subunits have a different extended conformation from the three other subunits in the closed channel, and identify several interactions on both pairs of subunit interfaces, and within the alpha subunits, which may be responsible for their "distorted" structures. The ACh-coordinating amino acid side-chains of the alpha subunits are far apart in the closed channel, indicating that a localised rearrangement, involving closure of loops B and C around the bound ACh molecule, occurs upon activation. A comparison of the structure of the alpha subunit with that of AChBP having ligand present, suggests how the localised rearrangement overcomes the distortions and initiates the rotational movements associated with opening of the channel. Both vestibules of the channel are strongly electronegative, providing a cation-stabilising environment at either entrance of the membrane pore. Access to the pore on the intracellular side is further influenced by narrow lateral windows, which would be expected to screen out electrostatically ions of the wrong charge and size.
- MRC Laboratory of Molecular Biology, Hills Road, Cambridge CB2 2QH, UK. mas@mrc-lmb.cam.ac.uk
Organizational Affiliation: