Crystal Structure of Homoserine Transacetylase from Haemophilus influenzae Reveals a New Family of alpha/beta-Hydrolases
Mirza, I.A., Nazi, I., Korczynska, M., Wright, G.D., Berghuis, A.M.(2005) Biochemistry 44: 15768-15773
- PubMed: 16313180
- DOI: https://doi.org/10.1021/bi051951y
- Primary Citation of Related Structures:
2B61 - PubMed Abstract:
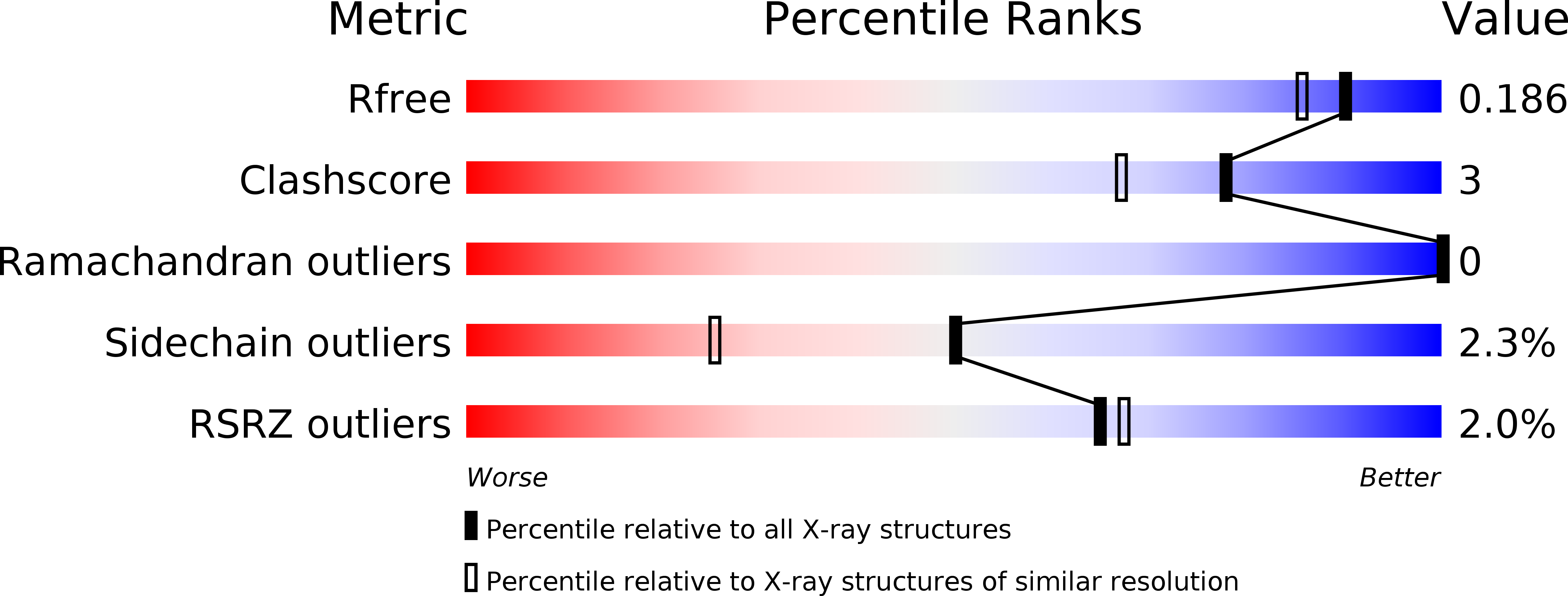
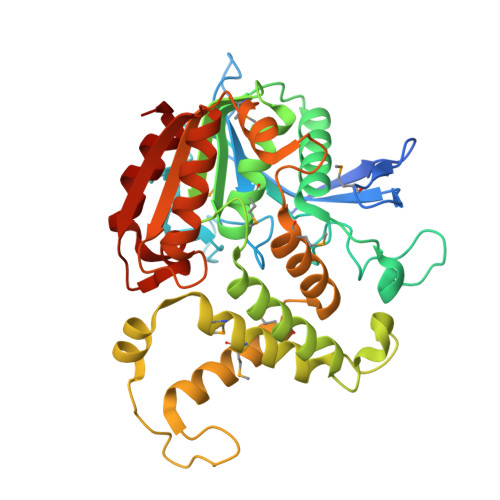
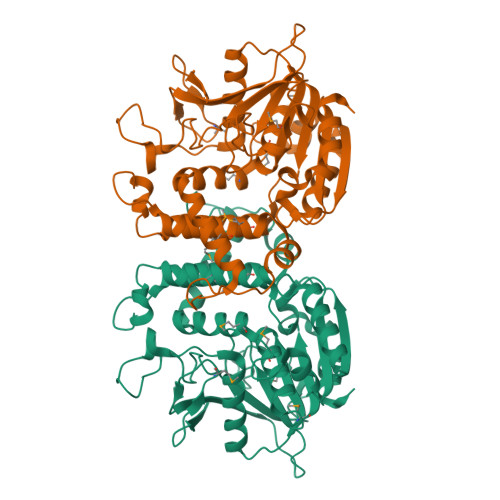
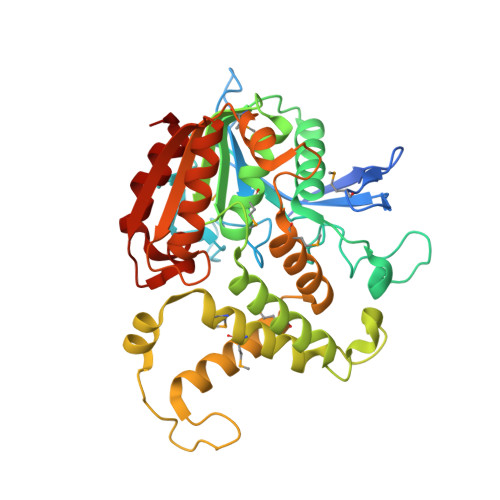
Homoserine transacetylase catalyzes one of the required steps in the biosynthesis of methionine in fungi and several bacteria. We have determined the crystal structure of homoserine transacetylase from Haemophilus influenzae to a resolution of 1.65 A. The structure identifies this enzyme to be a member of the alpha/beta-hydrolase structural superfamily. The active site of the enzyme is located near the end of a deep tunnel formed by the juxtaposition of two domains and incorporates a catalytic triad involving Ser143, His337, and Asp304. A structural basis is given for the observed double displacement kinetic mechanism of homoserine transacetylase. Furthermore, the properties of the tunnel provide a rationale for how homoserine transacetylase catalyzes a transferase reaction vs hydrolysis, despite extensive similarity in active site architecture to hydrolytic enzymes.
Organizational Affiliation:
Department of Biochemistry, McGill University, Montreal, Quebec, Canada H3A 1A4.