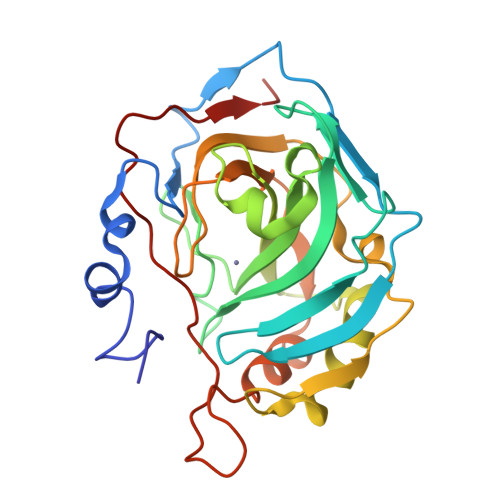
Production and X-ray crystallographic analysis of fully deuterated human carbonic anhydrase II.
Budayova-Spano, M., Fisher, S.Z., Dauvergne, M.T., Agbandje-McKenna, M., Silverman, D.N., Myles, D.A., McKenna, R.(2006) Acta Crystallogr Sect F Struct Biol Cryst Commun 62: 6-9
- PubMed: 16511248
- DOI: https://doi.org/10.1107/S1744309105038248
- Primary Citation of Related Structures:
2AX2 - PubMed Abstract:
Human carbonic anhydrase II (HCA II) is a zinc metalloenzyme that catalyzes the reversible hydration and dehydration of carbon dioxide and bicarbonate, respectively. The rate-limiting step in catalysis is the intramolecular transfer of a proton between the zinc-bound solvent (H2O/OH-) and the proton-shuttling residue His64. This distance (approximately 7.5 A) is spanned by a well defined active-site solvent network stabilized by amino-acid side chains (Tyr7, Asn62, Asn67, Thr199 and Thr200). Despite the availability of high-resolution (approximately 1.0 A) X-ray crystal structures of HCA II, there is currently no definitive information available on the positions and orientations of the H atoms of the solvent network or active-site amino acids and their ionization states. In preparation for neutron diffraction studies to elucidate this hydrogen-bonding network, perdeuterated HCA II has been expressed, purified, crystallized and its X-ray structure determined to 1.5 A resolution. The refined structure is highly isomorphous with hydrogenated HCA II, especially with regard to the active-site architecture and solvent network. This work demonstrates the suitability of these crystals for neutron macromolecular crystallography.
Organizational Affiliation:
European Molecular Biology Laboratory Grenoble Outstation, 6 Rue Jules Horowitz, 38042 Grenoble, France.