Engineering a Dimeric Caspase-9: A Re-Evaluation of the Induced Proximity Model for Caspase Activation
Chao, Y., Shiozaki, E.N., Srinivassula, S.M., Rigotti, D.J., Fairman, R., Shi, Y.(2005) PLoS Biol 3: 1079-1087
- PubMed: 15941357
- DOI: https://doi.org/10.1371/journal.pbio.0030183
- Primary Citation of Related Structures:
2AR9 - PubMed Abstract:
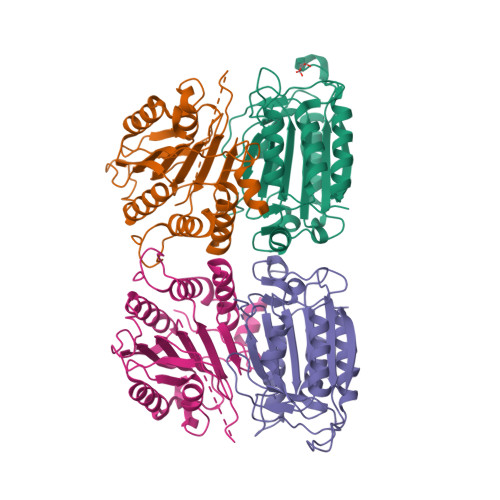
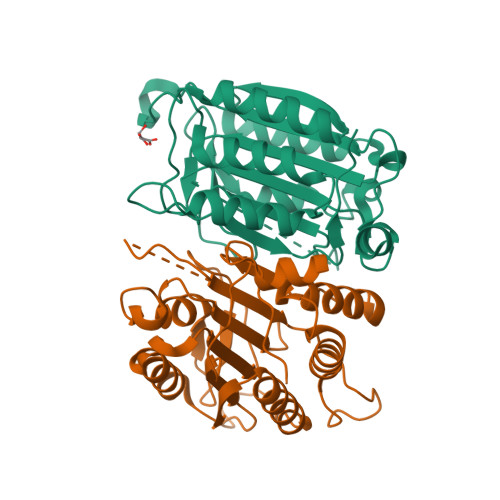
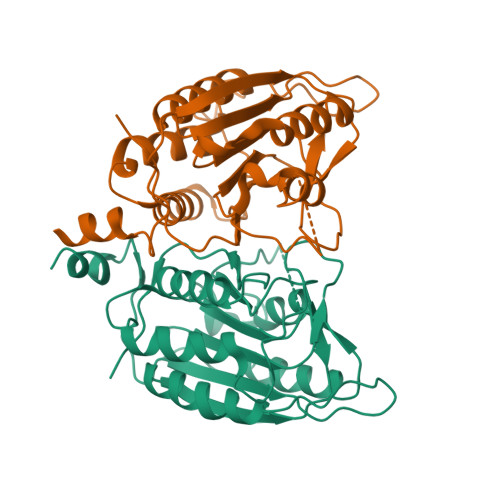
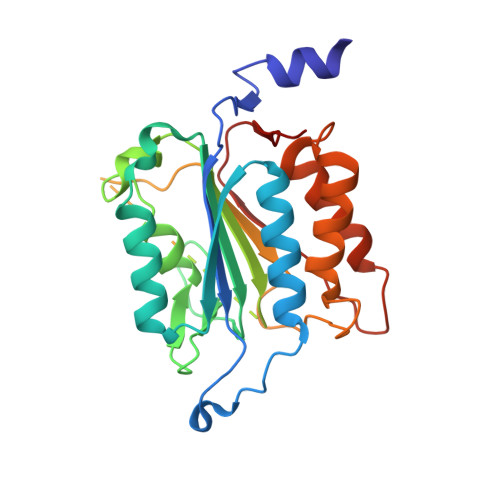
Caspases are responsible for the execution of programmed cell death (apoptosis) and must undergo proteolytic activation, in response to apoptotic stimuli, to function. The mechanism of initiator caspase activation has been generalized by the induced proximity model, which is thought to drive dimerization-mediated activation of caspases. The initiator caspase, caspase-9, exists predominantly as a monomer in solution. To examine the induced proximity model, we engineered a constitutively dimeric caspase-9 by relieving steric hindrance at the dimer interface. Crystal structure of the engineered caspase-9 closely resembles that of the wild-type (WT) caspase-9, including all relevant structural details and the asymmetric nature of two monomers. Compared to the WT caspase-9, this engineered dimer exhibits a higher level of catalytic activity in vitro and induces more efficient cell death when expressed. However, the catalytic activity of the dimeric caspase-9 is only a small fraction of that for the Apaf-1-activated caspase-9. Furthermore, in contrast to the WT caspase-9, the activity of the dimeric caspase-9 can no longer be significantly enhanced in an Apaf-1-dependent manner. These findings suggest that dimerization of caspase-9 may be qualitatively different from its activation by Apaf-1, and in conjunction with other evidence, posit an induced conformation model for the activation of initiator caspases.
Organizational Affiliation:
Department of Molecular Biology, Lewis Thomas Laboratory, Princeton University, Princeton, New Jersey, USA.