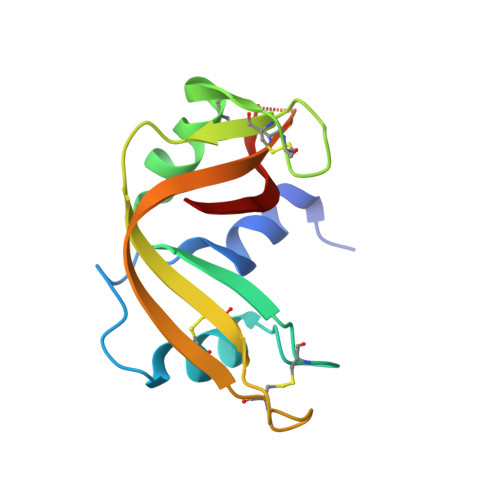
Amyloid-like fibrils of ribonuclease A with three-dimensional domain-swapped and native-like structure.
Sambashivan, S., Liu, Y., Sawaya, M.R., Gingery, M., Eisenberg, D.(2005) Nature 437: 266-269
- PubMed: 16148936
- DOI: https://doi.org/10.1038/nature03916
- Primary Citation of Related Structures:
2APQ - PubMed Abstract:
Amyloid or amyloid-like fibrils are elongated, insoluble protein aggregates, formed in vivo in association with neurodegenerative diseases or in vitro from soluble native proteins, respectively. The underlying structure of the fibrillar or 'cross-beta' state has presented long-standing, fundamental puzzles of protein structure. These include whether fibril-forming proteins have two structurally distinct stable states, native and fibrillar, and whether all or only part of the native protein refolds as it converts to the fibrillar state. Here we show that a designed amyloid-like fibril of the well-characterized enzyme RNase A contains native-like molecules capable of enzymatic activity. In addition, these functional molecular units are formed from a core RNase A domain and a swapped complementary domain. These findings are consistent with the zipper-spine model in which a cross-beta spine is decorated with three-dimensional domain-swapped functional units, retaining native-like structure.
Organizational Affiliation:
Howard Hughes Medical Institute, UCLA-DOE Institute for Genomics and Proteomics, Box 951570, UCLA, Los Angeles, California 90095-1570, USA.