Thrombomodulin-independent Activation of Protein C and Specificity of Hemostatically Active Snake Venom Serine Proteinases: CRYSTAL STRUCTURES OF NATIVE AND INHIBITED AGKISTRODON CONTORTRIX CONTORTRIX PROTEIN C ACTIVATOR.
Murakami, M.T., Arni, R.K.(2005) J Biological Chem 280: 39309-39315
- PubMed: 16162508
- DOI: https://doi.org/10.1074/jbc.M508502200
- Primary Citation of Related Structures:
2AIP, 2AIQ - PubMed Abstract:
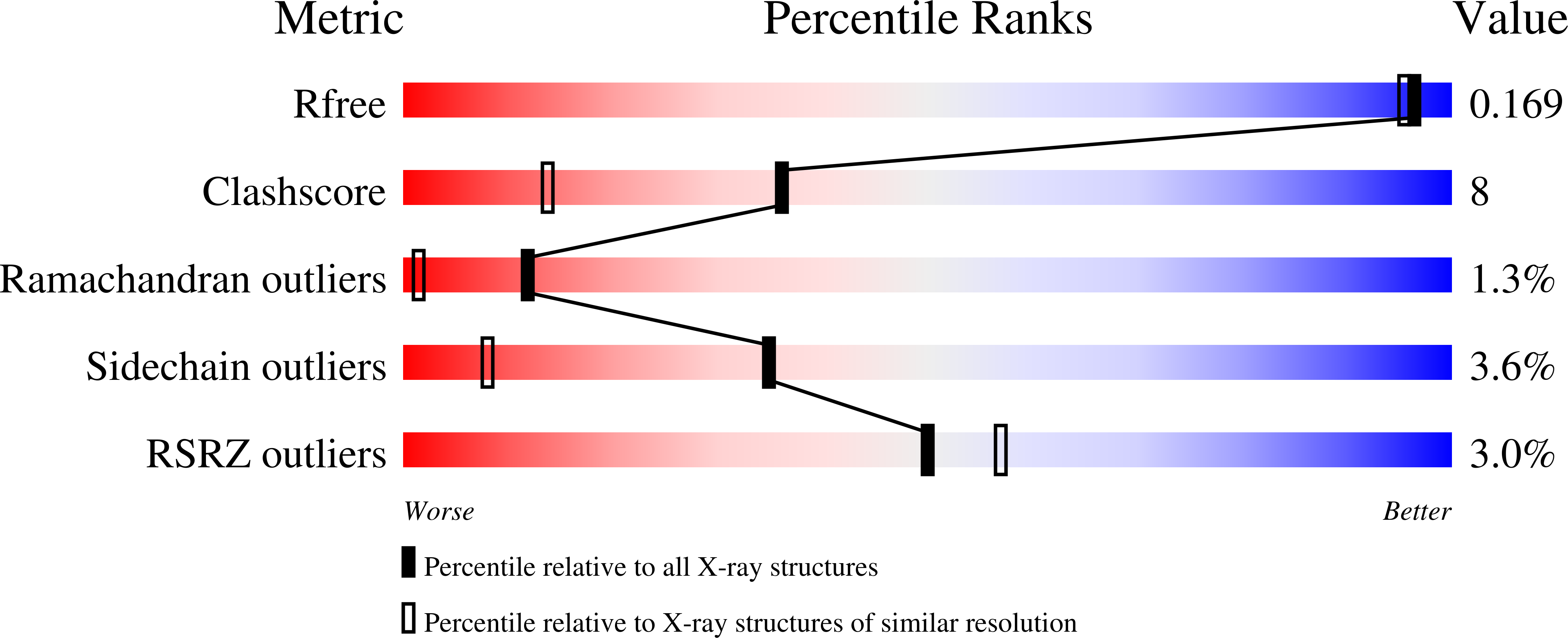
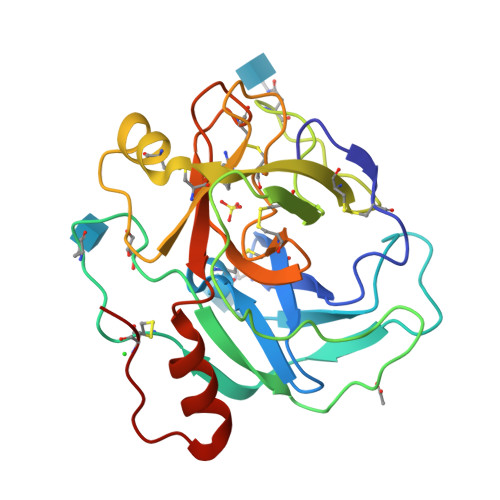
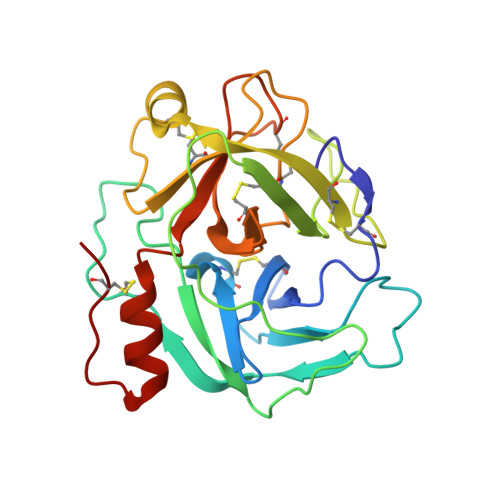
Protein C activation initiated by the thrombin-thrombomodulin complex forms the major physiological anticoagulant pathway. Agkistrodon contortrix contortrix protein C activator, a glycosylated single-chain serine proteinase, activates protein C without relying on thrombomodulin. The crystal structures of native and inhibited Agkistrodon contortrix contortrix protein C activator determined at 1.65 and 1.54 A resolutions, respectively, indicate the pivotal roles played by the positively charged belt and the strategic positioning of the three carbohydrate moieties surrounding the catalytic site in protein C recognition, binding, and activation. Structural changes in the benzamidine-inhibited enzyme suggest a probable function in allosteric regulation for the anion-binding site located in the C-terminal extension, which is fully conserved in snake venom serine proteinases, that preferentially binds Cl(1-) instead of SO(4)(2-).
Organizational Affiliation:
Biochemistry and Structural Biology Group, Department of Physics, IBILCE/UNESP, São José do Rio Preto, SP 15054-000, Brazil.