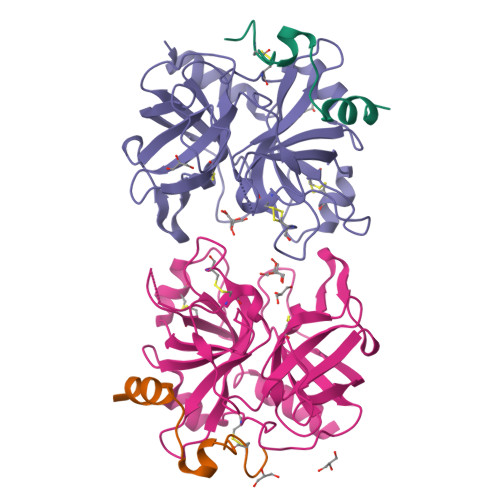
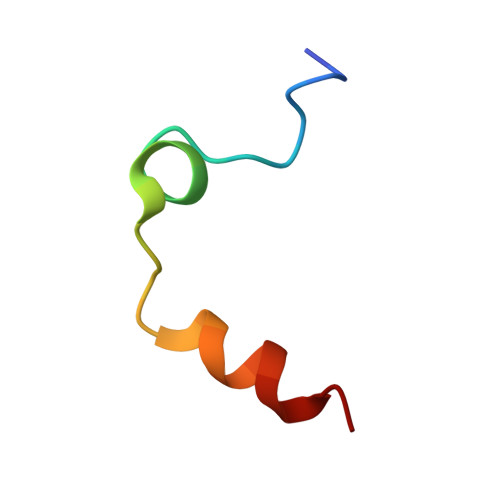
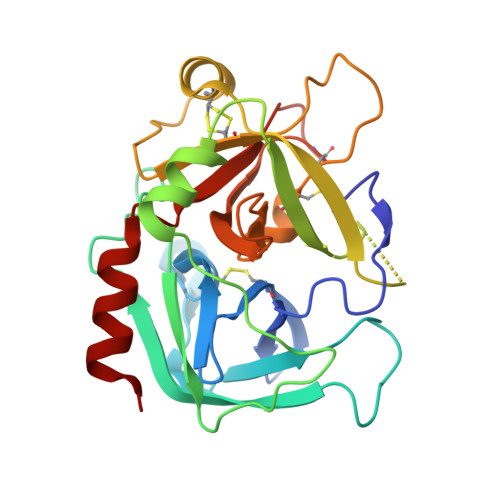
Crystal structure of wild-type human thrombin in the Na+-free state
Johnson, D.J.D., Adams, T.E., Li, W., Huntington, J.A.(2005) Biochem J 392: 21-28
- PubMed: 16201969
- DOI: https://doi.org/10.1042/BJ20051217
- Primary Citation of Related Structures:
2AFQ - PubMed Abstract:
Regulation of thrombin activity is critical for haemostasis and the prevention of thrombosis. Thrombin has several procoagulant substrates, including fibrinogen and platelet receptors, and essential cofactors for stimulating its own formation. However, thrombin is also capable of serving an anticoagulant function by activating protein C. The specificity of thrombin is primarily regulated by binding to the cofactor TM (thrombomodulin), but co-ordination of Na+ can also affect thrombin activity. The Na+-free form is often referred to as 'slow' because of reduced rates of cleavage of procoagulant substrates, but the slow form is still capable of rapid activation of protein C in the presence of TM. The molecular basis of the slow proteolytic activity of thrombin has remained elusive, in spite of two decades of solution studies and many published crystallographic structures. In the present paper, we report the first structure of wild-type unliganded human thrombin grown in the absence of co-ordinating Na+. The Na+-binding site is observed in a highly ordered position 6 A (1 A=0.1 nm) removed from that seen in the Na+-bound state. The movement of the Na+ loop results in non-catalytic hydrogen-bonding in the active site and blocking of the S1 and S2 substrate-binding pockets. Similar, if more dramatic, changes were observed in a previous structure of the constitutively slow thrombin variant E217K. The slow behaviour of thrombin in solutions devoid of Na+ can now be understood in terms of an equilibrium between an inert species, represented by the crystal structure described in the present paper, and an active form, where the addition of Na+ populates the active state.
Organizational Affiliation:
University of Cambridge, Department of Haematology, Division of Structural Medicine, Thrombosis Research Unit, Cambridge Institute for Medical Research, Wellcome Trust/MRC Building, Hills Road, Cambridge CB2 2XY, UK.