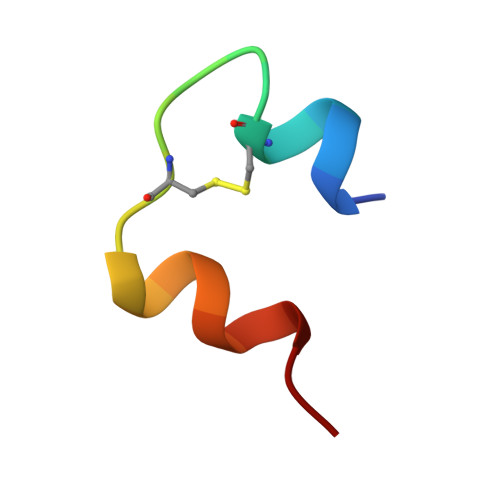
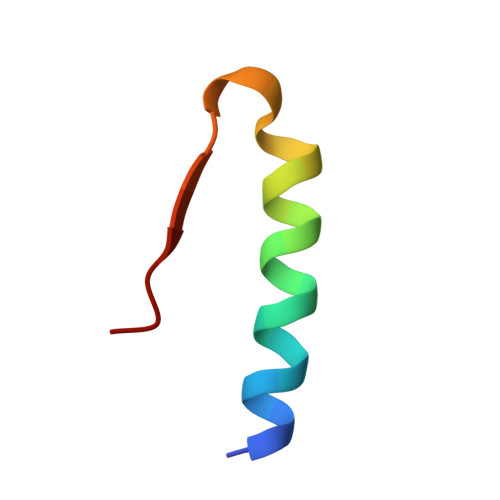
Crystal structure of a prolonged-acting insulin with albumin-binding properties.
Whittingham, J.L., Havelund, S., Jonassen, I.(1997) Biochemistry 36: 2826-2831
- PubMed: 9062110
- DOI: https://doi.org/10.1021/bi9625105
- Primary Citation of Related Structures:
1XDA - PubMed Abstract:
The fatty acid acylated insulin, Lys(B29)-tetradecanoyl, des-(B30) human insulin, has been crystallized and the structure determined by X-ray crystallography. The fatty acid substituent on residue B29 Lys binds reversibly to circulating albumin protein in vivo, and by this mechanism the hormone's action is prolonged. Crystals of the fatty acid insulin grow in space group R3, with two dimers in the asymmetric unit, and diffract to 1.8 A spacing. The structure has been solved by molecular replacement and refined using a maximum likelihood method. The crystal structure consists of R6 zinc insulin hexamers which contain phenol. The fatty acids can be seen bound between the hexamers, making specific interactions with the side chains of residue B1 Phe; however, the lysine side chains to which the fatty acids are covalently attached are mostly disordered. The mode of binding of the fatty acids appears to be determined by crystal packing, and whether or not they interact with the protein in this way in solution remains uncertain.
- Department of Chemistry, University of York, Heslington, England.
Organizational Affiliation: