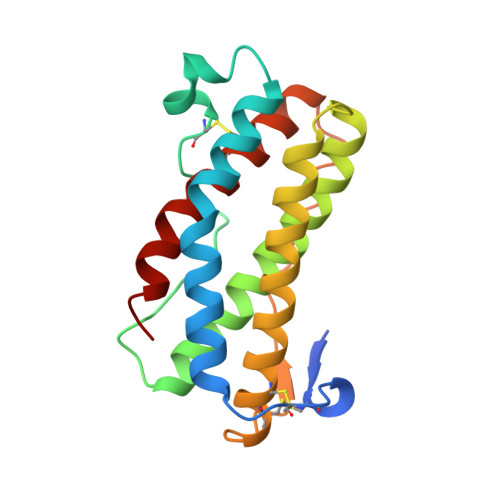
The crystal structure and biological function of leukemia inhibitory factor: implications for receptor binding.
Robinson, R.C., Grey, L.M., Staunton, D., Vankelecom, H., Vernallis, A.B., Moreau, J.F., Stuart, D.I., Heath, J.K., Jones, E.Y.(1994) Cell 77: 1101-1116
- PubMed: 8020098
- DOI: https://doi.org/10.1016/0092-8674(94)90449-9
- Primary Citation of Related Structures:
1LKI - PubMed Abstract:
The structure of murine leukemia inhibitory factor (LIF) has been determined by X-ray crystallography at 2.0 A resolution. The main chain fold conforms to the four alpha-helix bundle topology previously observed for several members of the hematopoietic cytokine family. Of these, LIF shows closest structural homology to granulocyte colony-stimulating factor and growth hormone (GH). Sequence alignments for the functionally related molecules oncostatin M and ciliary neurotrophic factor, when mapped to the LIF structure, indicate regions of conserved surface character. Analysis of the biological function and receptor specificity of a series of human-mouse LIF chimeras implicate two regions of receptor interaction that are located in the fourth helix and the preceding loop. A model for receptor binding based on the structure of the GH ligand-receptor complex requires additional, novel features to account for these data.
- Department of Biochemistry, University of Oxford, England.
Organizational Affiliation: