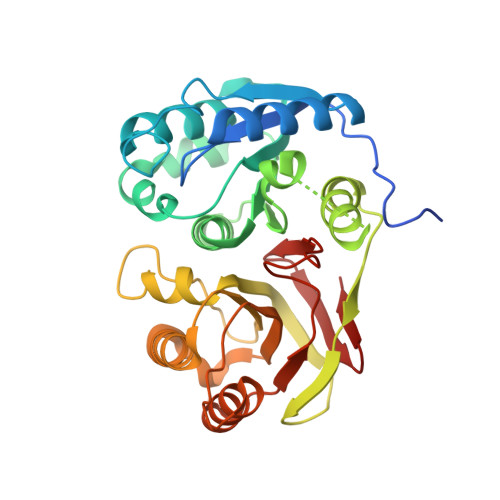
Pseudomonas aeruginosa LD-carboxypeptidase, a serine peptidase with a Ser-His-Glu triad and a nucleophilic elbow.
Korza, H.J., Bochtler, M.(2005) J Biol Chem 280: 40802-40812
- PubMed: 16162494
- DOI: https://doi.org/10.1074/jbc.M506328200
- Primary Citation of Related Structures:
1ZRS, 2AUM, 2AUN - PubMed Abstract:
LD-Carboxypeptidases (EC 3.4.17.13) are named for their ability to cleave amide bonds between l- and d-amino acids, which occur naturally in bacterial peptidoglycan. They are specific for the link between meso-diaminopimelic acid and d-alanine and therefore degrade GlcNAc-MurNAc tetrapeptides to the corresponding tripeptides. As only the tripeptides can be reused as peptidoglycan building blocks, ld-carboxypeptidases are thought to play a role in peptidoglycan recycling. Despite the pharmaceutical interest in peptidoglycan biosynthesis, the fold and catalytic type of ld-carboxypeptidases are unknown. Here, we show that a previously uncharacterized open reading frame in Pseudomonas aeruginosa has ld-carboxypeptidase activity and present the crystal structure of this enzyme. The structure shows that the enzyme consists of an N-terminal beta-sheet and a C-terminal beta-barrel domain. At the interface of the two domains, Ser(115) adopts a highly strained conformation in the context of a strand-turn-helix motif that is similar to the "nucleophilic elbow" in alphabeta-hydrolases. Ser(115) is hydrogen-bonded to a histidine residue, which is oriented by a glutamate residue. All three residues, which occur in the order Ser-Glu-His in the amino acid sequence, are strictly conserved in naturally occurring ld-carboxypeptidases and cannot be mutated to alanines without loss of activity. We conclude that ld-carboxypeptidases are serine peptidases with Ser-His-Glu catalytic triads.
Organizational Affiliation:
International Institute of Molecular and Cell Biology, Ulica Trojdena 4, 02-109 Warsaw, Poland.