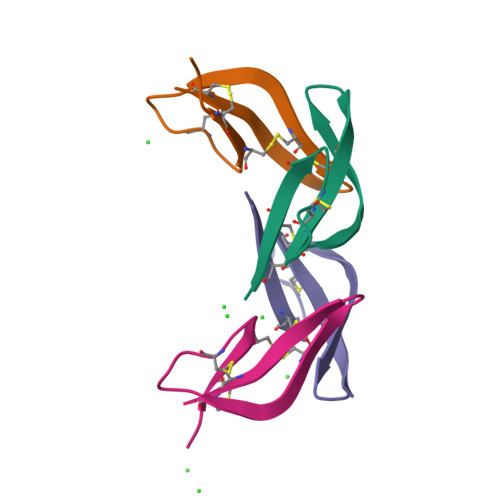
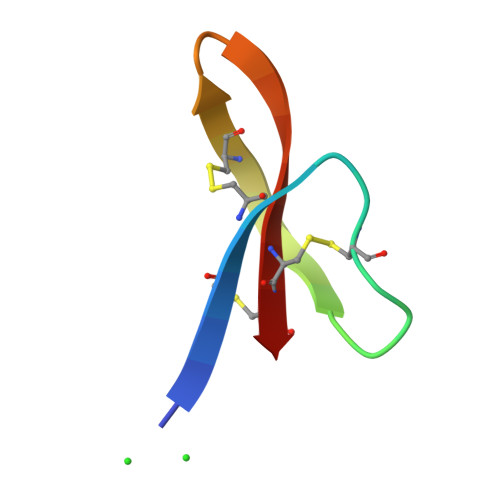
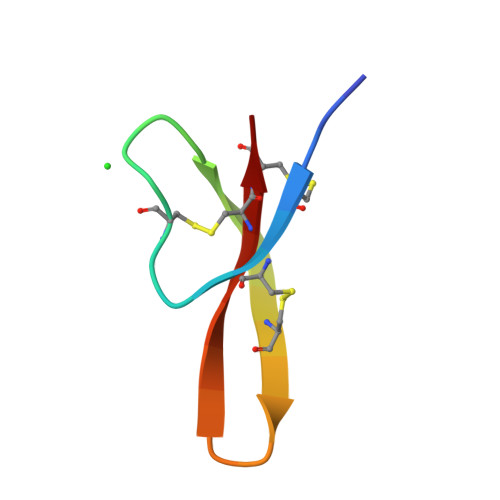
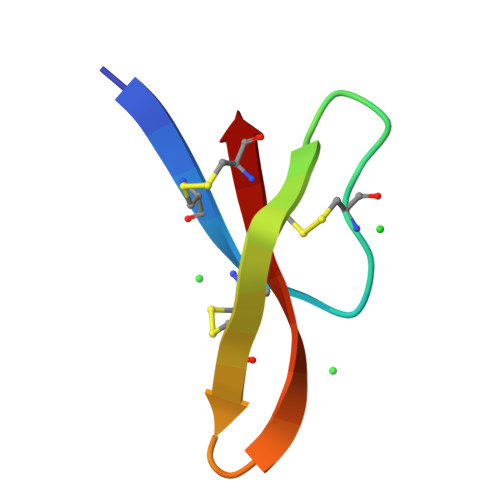
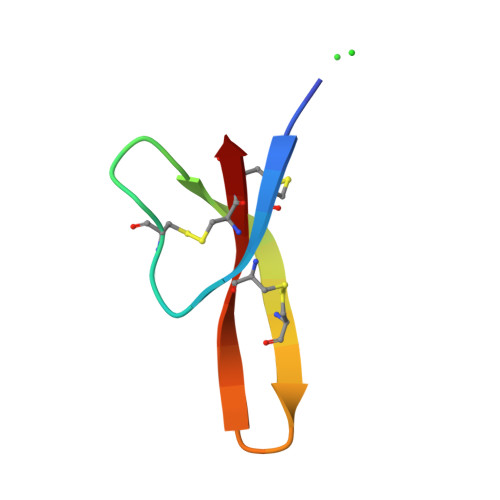
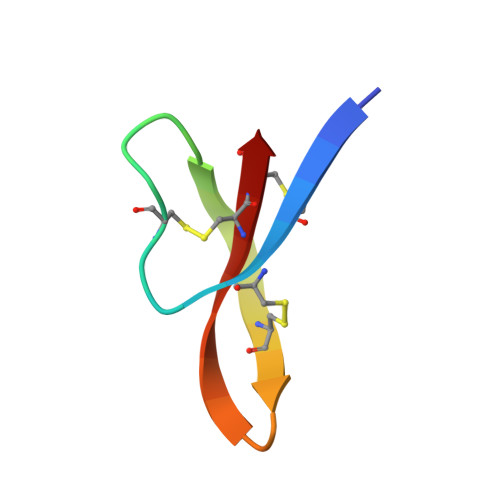
Crystal structures of human {alpha}-defensins HNP4, HD5, and HD6.
Szyk, A., Wu, Z., Tucker, K., Yang, D., Lu, W., Lubkowski, J.(2006) Protein Sci 15: 2749-2760
- PubMed: 17088326
- DOI: https://doi.org/10.1110/ps.062336606
- Primary Citation of Related Structures:
1ZMM, 1ZMP, 1ZMQ - PubMed Abstract:
Six alpha-defensins have been found in humans. These small arginine-rich peptides play important roles in various processes related to host defense, being the effectors and regulators of innate immunity as well as enhancers of adoptive immune responses. Four defensins, called neutrophil peptides 1 through 4, are stored primarily in polymorphonuclear leukocytes. Major sites of expression of defensins 5 and 6 are Paneth cells of human small intestine. So far, only one structure of human alpha-defensin (HNP3) has been reported, and the properties of the intestine defensins 5 and 6 are particularly poorly understood. In this report, we present the high-resolution X-ray structures of three human defensins, 4 through 6, supplemented with studies of their antimicrobial and chemotactic properties. Despite only modest amino acid sequence identity, all three defensins share their tertiary structures with other known alpha- and beta-defensins. Like HNP3 but in contrast to murine or rabbit alpha-defensins, human defensins 4-6 form characteristic dimers. Whereas antimicrobial and chemotactic activity of HNP4 is somewhat comparable to that of other human neutrophil defensins, neither of the intestinal defensins appears to be chemotactic, and for HD6 also an antimicrobial activity has yet to be observed. The unusual biological inactivity of HD6 may be associated with its structural properties, somewhat standing out when compared with other human alpha-defensins. The strongest cationic properties and unique distribution of charged residues on the molecular surface of HD5 may be associated with its highest bactericidal activity among human alpha-defensins.
- Macromolecular Assembly Structure and Cell Signaling Section, National Cancer Institute, Frederick, Maryland 21702, USA.
Organizational Affiliation: