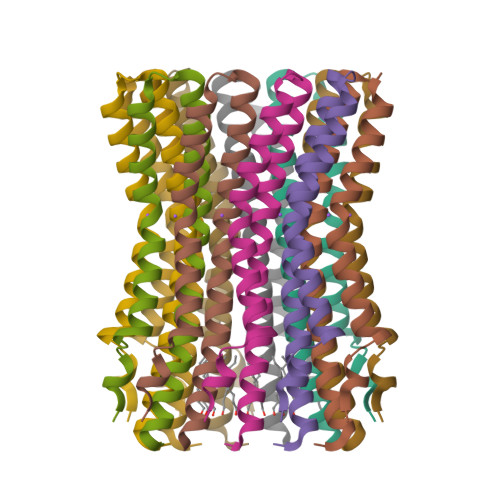
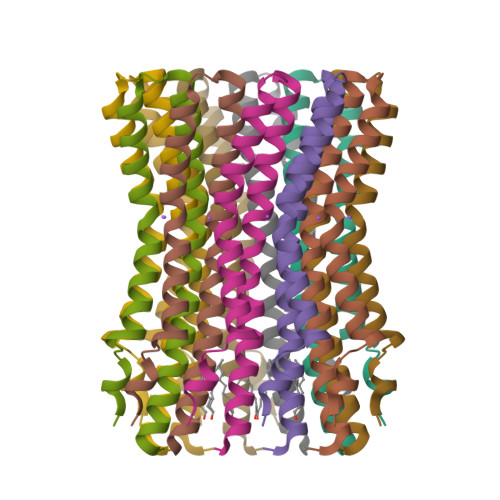
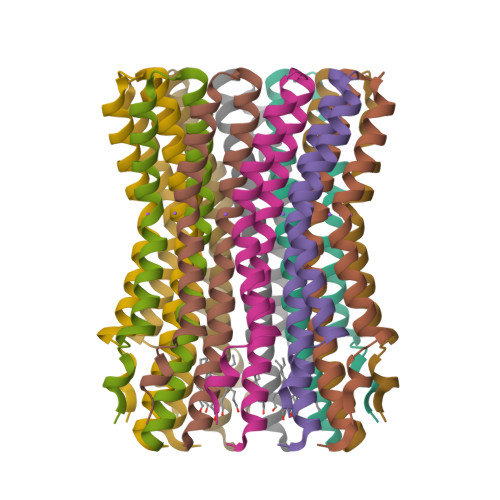
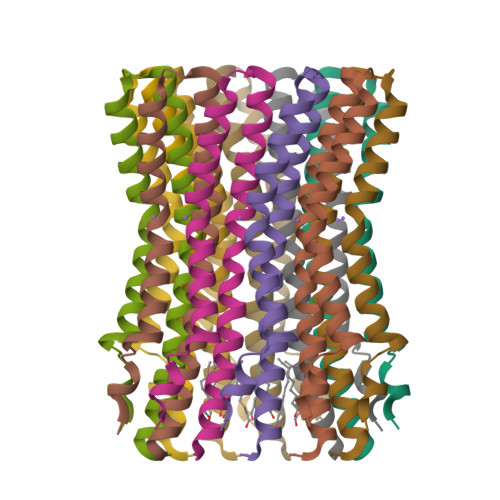
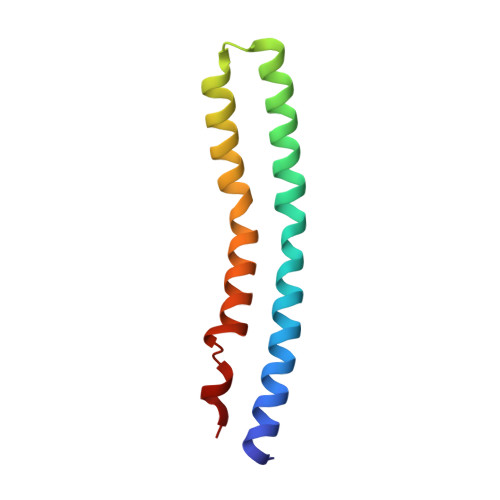
Structure of the rotor ring of F-Type Na+-ATPase from Ilyobacter tartaricus.
Meier, T., Polzer, P., Diederichs, K., Welte, W., Dimroth, P.(2005) Science 308: 659-662
- PubMed: 15860619
- DOI: https://doi.org/10.1126/science.1111199
- Primary Citation of Related Structures:
1YCE - PubMed Abstract:
In the crystal structure of the membrane-embedded rotor ring of the sodium ion-translocating adenosine 5'-triphosphate (ATP) synthase of Ilyobacter tartaricus at 2.4 angstrom resolution, 11 c subunits are assembled into an hourglass-shaped cylinder with 11-fold symmetry. Sodium ions are bound in a locked conformation close to the outer surface of the cylinder near the middle of the membrane. The structure supports an ion-translocation mechanism in the intact ATP synthase in which the binding site converts from the locked conformation into one that opens toward subunit a as the rotor ring moves through the subunit a/c interface.
Organizational Affiliation:
Institut für Mikrobiologie, Eidgenössische Technische Hochschule (ETH), Zürich Hönggerberg, Wolfgang-Pauli-Str. 10, CH-8093 Zürich, Switzerland.