Novel Anion-independent Iron Coordination by Members of a Third Class of Bacterial Periplasmic Ferric Ion-binding Proteins
Shouldice, S.R., McRee, D.E., Dougan, D.R., Tari, L.W., Schryvers, A.B.(2005) J Biological Chem 280: 5820-5827
- PubMed: 15576371
- DOI: https://doi.org/10.1074/jbc.M411238200
- Primary Citation of Related Structures:
1XVX, 1XVY - PubMed Abstract:
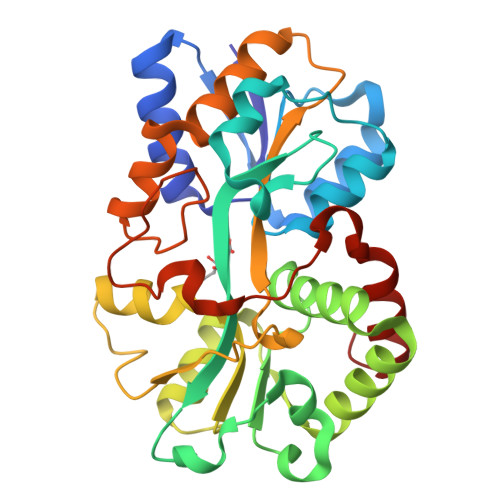
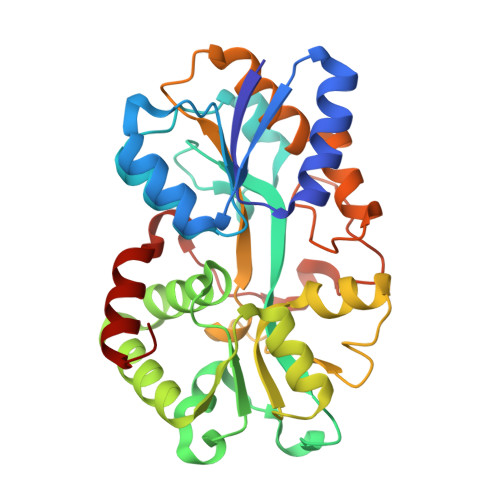
The uptake of the element iron is vital for the survival of most organisms. Numerous pathogenic Gram-negative bacteria utilize a periplasm-to-cytosol ATP-binding cassette transport pathway to transport this essential atom in to the cell. In this study, we investigated the Yersinia enterocolitica (YfuA) and Serratia marcescens (SfuA) iron-binding periplasmic proteins. We have determined the 1.8-angstroms structures of iron-loaded (YfuA) and iron-free (SfuA) forms of this class of proteins. Although the sequence of these proteins varies considerably from the other members of the transferrin structural superfamily, they adopt the same three-dimensional fold. The iron-loaded YfuA structure illustrates the unique nature of this new class of proteins in that they are able to octahedrally coordinate the ferric ion in the absence of a bound anion. The iron-free SfuA structure contains a bound citrate anion in the iron-binding cleft that tethers the N- and C-terminal domains of the apo protein and stabilizes the partially open structure.
Organizational Affiliation:
Department of Microbiology and Infectious Diseases, University of Calgary, Calgary, Alberta T2N 4N1, Canada.