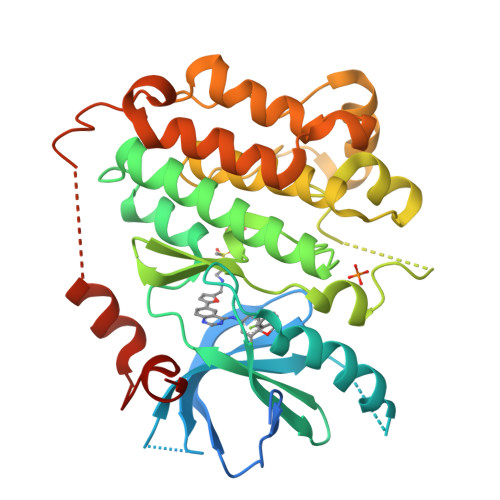
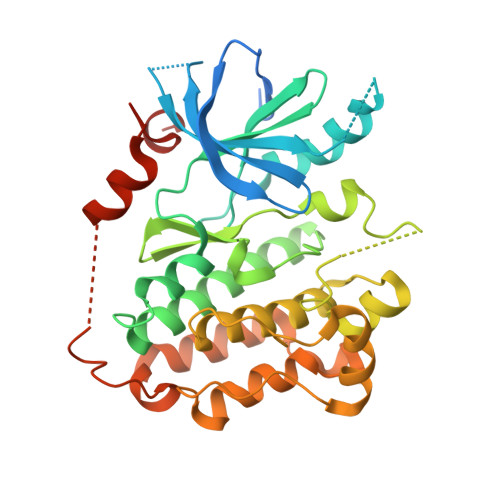
A unique structure for epidermal growth factor receptor bound to GW572016 (Lapatinib): relationships among protein conformation, inhibitor off-rate, and receptor activity in tumor cells.
Wood, E.R., Truesdale, A.T., McDonald, O.B., Yuan, D., Hassell, A., Dickerson, S.H., Ellis, B., Pennisi, C., Horne, E., Lackey, K., Alligood, K.J., Rusnak, D.W., Gilmer, T.M., Shewchuk, L.(2004) Cancer Res 64: 6652-6659
- PubMed: 15374980
- DOI: https://doi.org/10.1158/0008-5472.CAN-04-1168
- Primary Citation of Related Structures:
1XKK - PubMed Abstract:
GW572016 (Lapatinib) is a tyrosine kinase inhibitor in clinical development for cancer that is a potent dual inhibitor of epidermal growth factor receptor (EGFR, ErbB-1) and ErbB-2. We determined the crystal structure of EGFR bound to GW572016. The compound is bound to an inactive-like conformation of EGFR that is very different from the active-like structure bound by the selective EGFR inhibitor OSI-774 (Tarceva) described previously. Surprisingly, we found that GW572016 has a very slow off-rate from the purified intracellular domains of EGFR and ErbB-2 compared with OSI-774 and another EGFR selective inhibitor, ZD-1839 (Iressa). Treatment of tumor cells with these inhibitors results in down-regulation of receptor tyrosine phosphorylation. We evaluated the duration of the drug effect after washing away free compound and found that the rate of recovery of receptor phosphorylation in the tumor cells reflected the inhibitor off-rate from the purified intracellular domain. The slow off-rate of GW572016 correlates with a prolonged down-regulation of receptor tyrosine phosphorylation in tumor cells. The differences in the off-rates of these drugs and the ability of GW572016 to inhibit ErbB-2 can be explained by the enzyme-inhibitor structures.
Organizational Affiliation:
Department of Computational, Analytical and Structural Sciences, GlaxoSmithKline, Inc., Research Triangle Park, North Carolina 27709, USA. ew39216@gsk.com