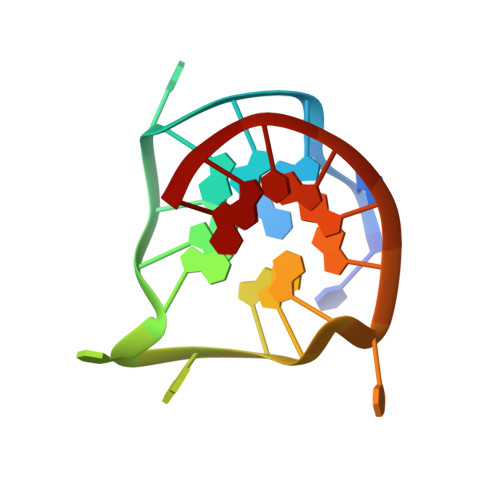
Solution structure of the biologically relevant G-Quadruplex element in the human c-MYC promoter. Implications for G-quadruplex stabilization.
Ambrus, A., Chen, D., Dai, J., Jones, R.A., Yang, D.(2005) Biochemistry 44: 2048-2058
- PubMed: 15697230
- DOI: https://doi.org/10.1021/bi048242p
- Primary Citation of Related Structures:
1XAV - PubMed Abstract:
The nuclease hypersensitivity element III(1) (NHE III(1)) of the c-MYC promoter strongly controls the transcriptional activity of the c-MYC oncogene. The purine-rich strand of the NHE III(1) element has been shown to be a silencer element for c-MYC transcription upon formation of a G-quadruplex structure. We have determined the predominant G-quadruplex structure of this silencer element in potassium solution by NMR. The G-quadruplex structure adopts an intramolecular parallel-stranded quadruplex conformation with three guanine tetrads and three side loops, including two single-nucleotide side loops and one double-nucleotide side loop, that connect the four guanine strands. The three side loops are very stable and well-defined. The 3'-flanking sequence forms a stable fold-back stacking conformation capping the top end of the G-quadruplex structure. The 5'-flanking A and G bases cap the bottom end of the G-quadruplex, with the adenine stacking very well with the bottom tetrad. This paper reports the first solution structure of a G-quadruplex found to form in the promoter region of an oncogene (c-MYC). This G-quadruplex structure is extremely stable, with a similar melting temperature (>85 degrees C) to that of the wild-type 27-mer purine-rich NHE III(1) sequence of the c-MYC promoter. This predominant quadruplex structure has been shown to be biologically relevant, and the structural information revealed in this research provides an important basis for the design of new drug candidates that specifically target the c-MYC G-quadruplex structure and modulate gene expression.
- College of Pharmacy, The University of Arizona, 1703 East Mabel Street, Tucson, Arizona 85721, USA.
Organizational Affiliation: