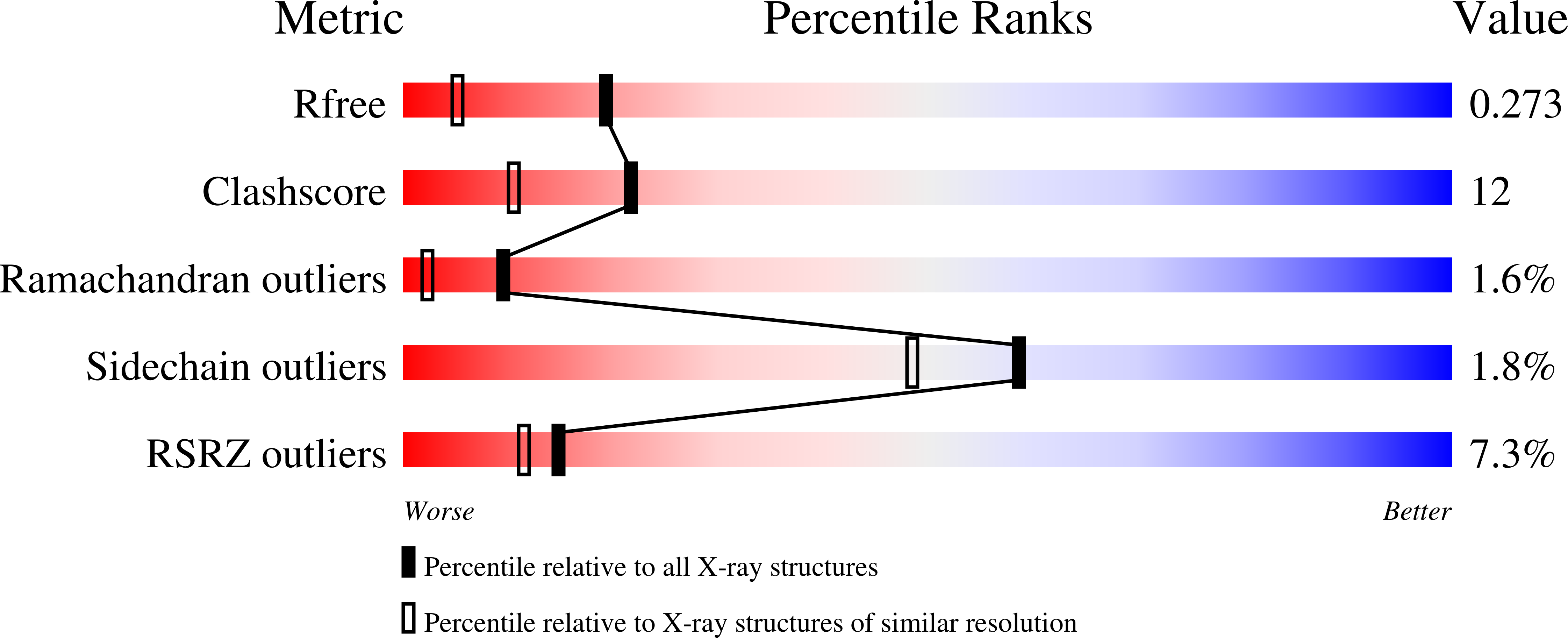
Probing the DNA kink structure induced by the hyperthermophilic chromosomal protein Sac7d
Chen, C.-Y., Ko, T.-P., Lin, T.-W., Chou, C.-C., Chen, C.-J., Wang, A.H.-J.(2005) Nucleic Acids Res 33: 430-438
- PubMed: 15653643
- DOI: https://doi.org/10.1093/nar/gki191
- Primary Citation of Related Structures:
1WTO, 1WTP, 1WTQ, 1WTR, 1WTV, 1WTW, 1WTX, 1XYI - PubMed Abstract:
Sac7d, a small, abundant, sequence-general DNA-binding protein from the hyperthermophilic archaeon Sulfolobus acidocaldarius, causes a single-step sharp kink in DNA (approximately 60 degrees) via the intercalation of both Val26 and Met29. These two amino acids were systematically changed in size to probe their effects on DNA kinking. Eight crystal structures of five Sac7d mutant-DNA complexes have been analyzed. The DNA-binding pattern of the V26A and M29A single mutants is similar to that of the wild-type, whereas the V26A/M29A protein binds DNA without side chain intercalation, resulting in a smaller overall bending (approximately 50 degrees). The M29F mutant inserts the Phe29 side chain orthogonally to the C2pG3 step without stacking with base pairs, inducing a sharp kink (approximately 80 degrees). In the V26F/M29F-GCGATCGC complex, Phe26 intercalates deeply into DNA bases by stacking with the G3 base, whereas Phe29 is stacked on the G15 deoxyribose, in a way similar to those used by the TATA box-binding proteins. All mutants have reduced DNA-stabilizing ability, as indicated by their lower T m values. The DNA kink patterns caused by different combinations of hydrophobic side chains may be relevant in understanding the manner by which other minor groove-binding proteins interact with DNA.
Organizational Affiliation:
Institute of Biological Chemistry Taipei 115, Taiwan.