Crystal Structure of the Terminal Oxygenase Component of Cumene Dioxygenase from Pseudomonas fluorescens IP01
Dong, X., Fushinobu, S., Fukuda, E., Terada, T., Nakamura, S., Shimizu, K., Nojiri, H., Omori, T., Shoun, H., Wakagi, T.(2005) J Bacteriol 187: 2483-2490
- PubMed: 15774891
- DOI: https://doi.org/10.1128/JB.187.7.2483-2490.2005
- Primary Citation of Related Structures:
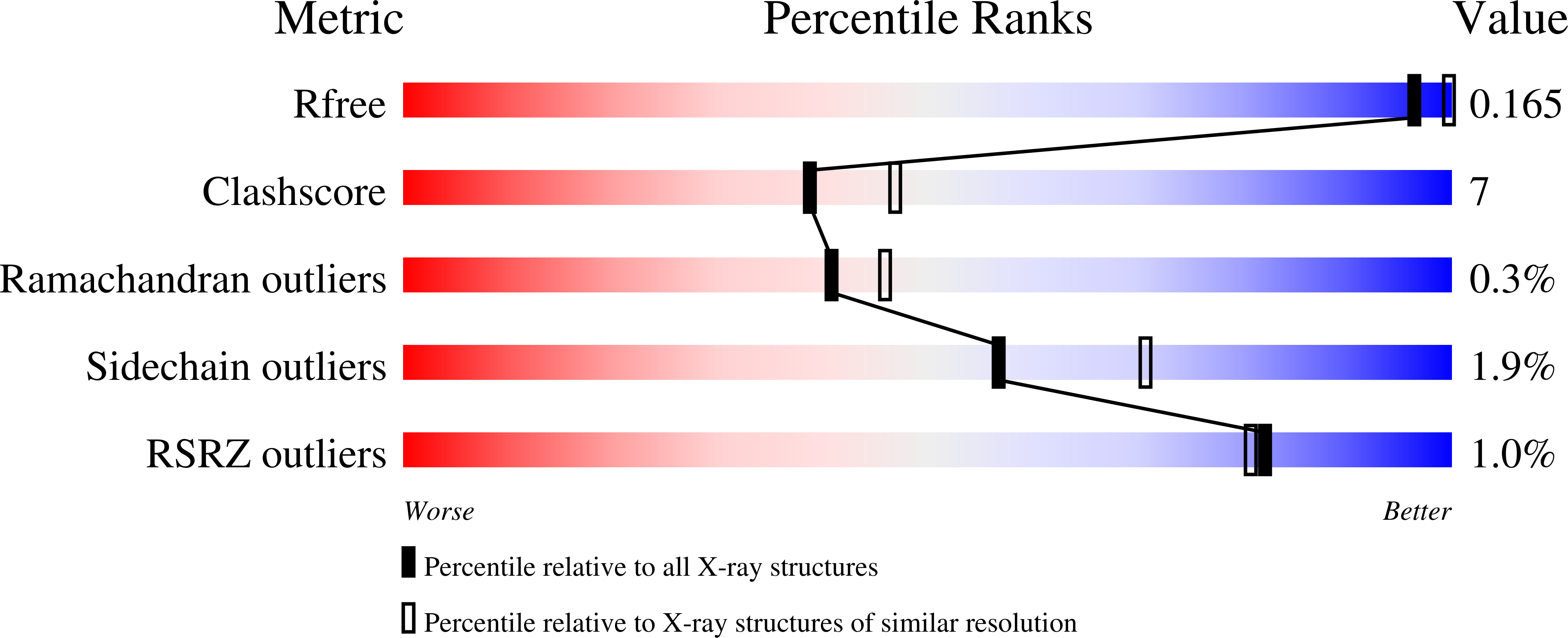
1WQL - PubMed Abstract:
The crystal structure of the terminal component of the cumene dioxygenase multicomponent enzyme system of Pseudomonas fluorescens IP01 (CumDO) was determined at a resolution of 2.2 A by means of molecular replacement by using the crystal structure of the terminal oxygenase component of naphthalene dioxygenase from Pseudomonas sp. strain NCIB 9816-4 (NphDO). The ligation of the two catalytic centers of CumDO (i.e., the nonheme iron and Rieske [2Fe-2S] centers) and the bridging between them in neighboring catalytic subunits by hydrogen bonds through a single amino acid residue, Asp231, are similar to those of NphDO. An unidentified external ligand, possibly dioxygen, was bound at the active site nonheme iron. The entrance to the active site of CumDO is different from the entrance to the active site of NphDO, as the two loops forming the lid exhibit great deviation. On the basis of the complex structure of NphDO, a biphenyl substrate was modeled in the substrate-binding pocket of CumDO. The residues surrounding the modeled biphenyl molecule include residues that have already been shown to be important for its substrate specificity by a number of engineering studies of biphenyl dioxygenases.
Organizational Affiliation:
Department of Biotechnology, The University of Tokyo, 1-1-1 Yayoi, Bunkyo-ku, Tokyo 113-8657, Japan.