X-ray structural studies of Mycobacterium tuberculosis RRF and a comparative study of RRFs of known structure. Molecular plasticity and biological implications
Saikrishnan, K., Kalapala, S.K., Varshney, U., Vijayan, M.(2005) J Mol Biol 345: 29-38
- PubMed: 15567408
- DOI: https://doi.org/10.1016/j.jmb.2004.10.034
- Primary Citation of Related Structures:
1WQF, 1WQG, 1WQH - PubMed Abstract:
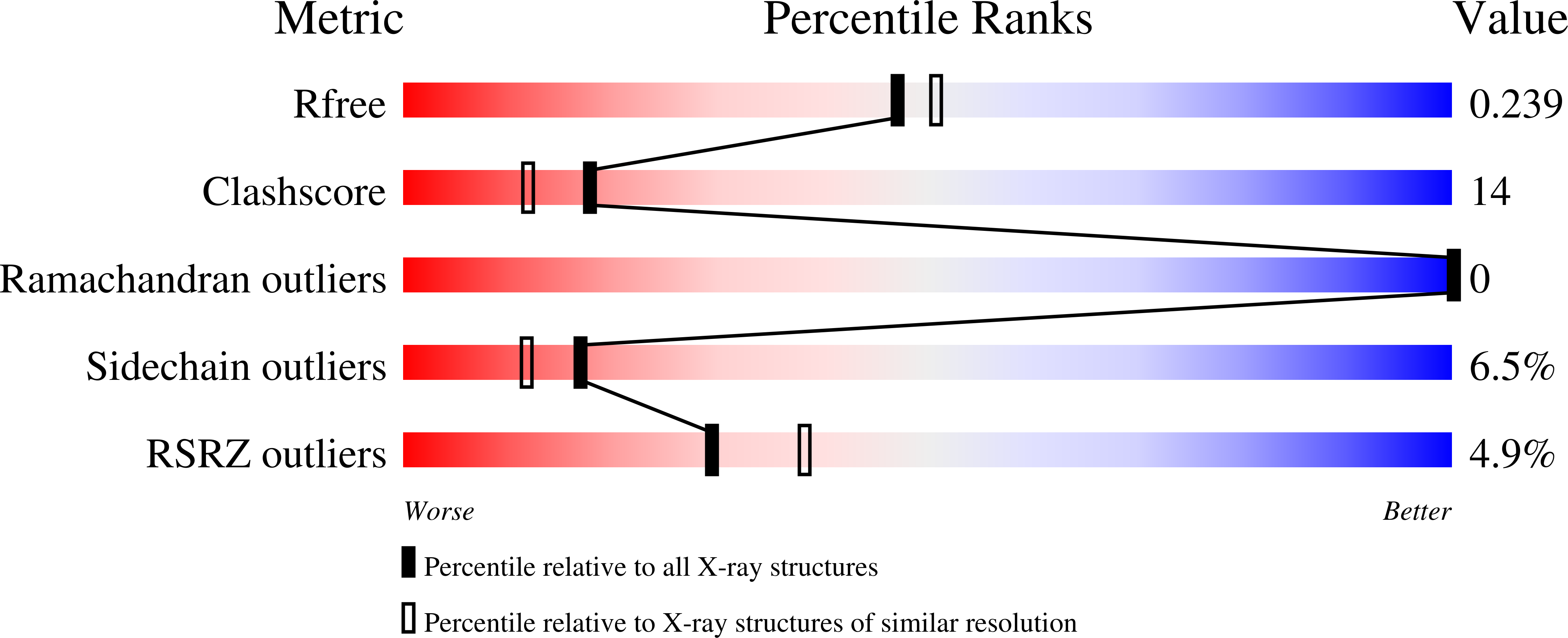
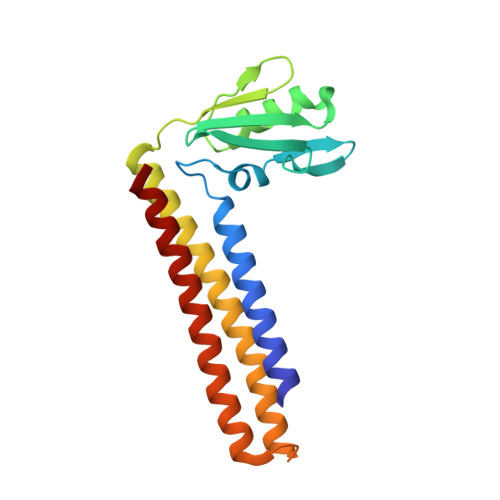
The crystal structure of Mycobacterium tuberculosis ribosome recycling factor has been determined and refined against three X-ray diffraction data sets, two collected at room temperature and the other at 100K. The two room-temperature data sets differ in the radiation damage suffered by the crystals before the data used for processing were collected. A comparison between the structures refined against the two data sets indicates the possibility of radiation-induced conformational change. The L-shaped molecule is composed of a long three-helix bundle domain (domain I) and a globular domain (domain II) connected by a linker region. The main difference between the room-temperature structure and the low temperature structure is in the rotation of domain II about an axis close to its libration axis. This observation and a detailed comparative study of ribosome recycling factors (RRFs) of known structures led to an elaboration of the present understanding of the structural variability of RRF. The variability involves a change in the angle between the two arms of the molecule, a rotation of domain II in a plane nearly perpendicular to the axis of the helix bundle and an internal rotation of domain II. Furthermore, the domains and the linker could be delineated into fixed and variable regions in a physically meaningful manner. The relative mobility of the domains of the molecule in the crystal structure appears to be similar to that in the ribosome--RRF complex. That permits a meaningful discussion of the structural features of RRF in terms of ribosome--RRF interactions. The structure also provides insights into the results of inter-species complementation studies.
Organizational Affiliation:
Molecular Biophysics Unit, Indian Institute of Science, Bangalore 560012, India.