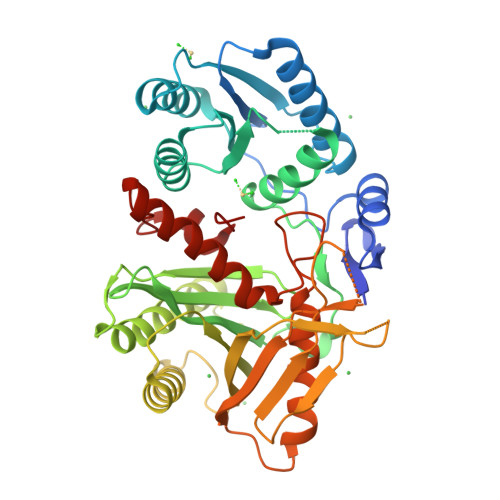
Crystal Structure of a Piwi Protein Suggests Mechanisms for Sirna Recognition and Slicer Activity
Parker, J.S., Roe, S.M., Barford, D.(2004) EMBO J 23: 4727
- PubMed: 15565169
- DOI: https://doi.org/10.1038/sj.emboj.7600488
- Primary Citation of Related Structures:
1W9H - PubMed Abstract:
RNA silencing regulates gene expression through mRNA degradation, translation repression and chromatin remodelling. The fundamental engines of RNA silencing are RISC and RITS complexes, whose common components are 21-25 nt RNA and an Argonaute protein containing a PIWI domain of unknown function. The crystal structure of an archaeal Piwi protein (AfPiwi) is organised into two domains, one resembling the sugar-binding portion of the lac repressor and another with similarity to RNase H. Invariant residues and a coordinated metal ion lie in a pocket that surrounds the conserved C-terminus of the protein, defining a key functional region in the PIWI domain. Furthermore, two Asp residues, conserved in the majority of Argonaute sequences, align spatially with the catalytic Asp residues of RNase H-like catalytic sites, suggesting that in eukaryotic Argonaute proteins the RNase H-like domain may possess nuclease activity. The conserved region around the C-terminus of the PIWI domain, which is required for small interfering RNA (siRNA) binding to AfPiwi, may function as the receptor site for the obligatory 5' phosphate of siRNAs, thereby specifying the cleavage position of the target mRNA.
Organizational Affiliation:
Section of Structural Biology, The Institute of Cancer Research, Chester Beatty Laboratories, London, UK.