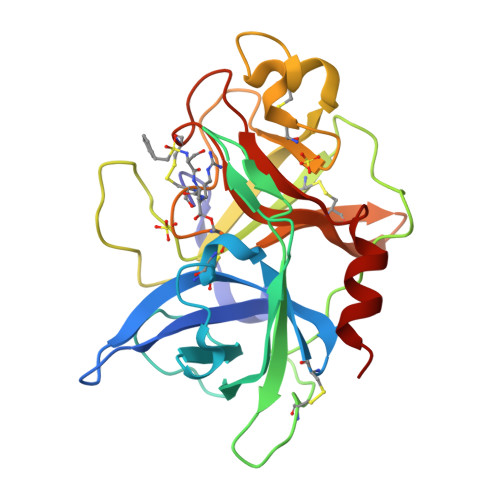
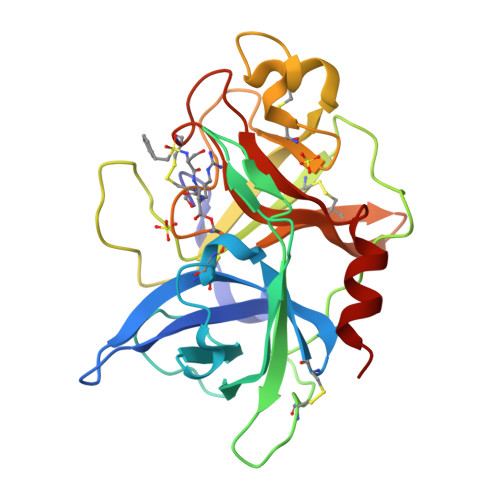
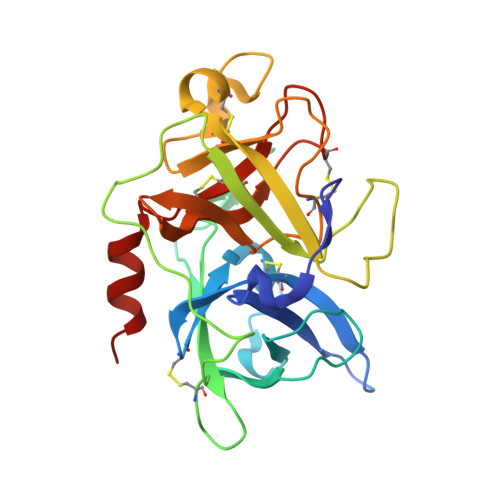
Crystals of Urokinase Type Plasminogen Activator Complexes Reveal the Binding Mode of Peptidomimetic Inhibitors.
Zeslawska, E., Jacob, U., Schweinitz, A., Coombs, G., Bode, W., Madison, E.(2003) J Mol Biology 328: 109
- PubMed: 12684001
- DOI: https://doi.org/10.1016/s0022-2836(03)00267-5
- Primary Citation of Related Structures:
1W0Z, 1W10, 1W11, 1W12, 1W13, 1W14 - PubMed Abstract:
Urokinase type plasminogen activator (uPA), a trypsin-like serine proteinase, plays an important role in normal tissue re-modelling, cell adhesion, and cell motility. In addition, studies utilizing normal animals and potent, selective uPA inhibitors or genetically modified mice that lack functional uPA genes have demonstrated that uPA can significantly enhance tumor initiation, growth, progression and metastasis, strongly suggesting that this enzyme may be a promising anti-cancer target. We have investigated the structure-activity relationship (SAR) of peptidomimetic inhibitors of uPA and solved high resolution X-ray structures of key, lead small molecule inhibitors (e.g. phenethylsulfonamidino(P4)-D-seryl(P3)-L-alanyl(P2)-L-argininal(P1) and derivatives thereof) in complex with the uPA proteinase domain. These potent inhibitors are highly selective for uPA. The non-natural D-seryl residue present at the P3 position in these inhibitors contributes substantially to both potency and selectivity because, due to its D-configuration, its side-chain binds in the S4 pocket to interact with the uPA unique residues Leu97b and His99. Additional potency and selectivity can be achieved by optimizing the inhibitor P4 residue to bind a pocket, known as S1sub or S1beta, that is adjacent to the primary specificity pocket of uPA.
Organizational Affiliation:
Pedagogical University, Department of Chemistry, Podchorazych 2, 30-084 Cracow, Poland.