The First Crystal Structure of Hyperthermostable NAD-dependent Glutamate Dehydrogenase from Pyrobaculum islandicum
Bhuiya, M.W., Sakuraba, H., Ohshima, T., Imagawa, T., Katunuma, N., Tsuge, H.(2005) J Mol Biology 345: 325-337
- PubMed: 15571725
- DOI: https://doi.org/10.1016/j.jmb.2004.10.063
- Primary Citation of Related Structures:
1V9L - PubMed Abstract:
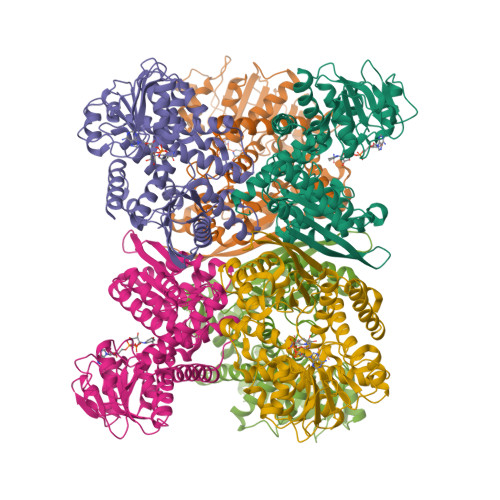
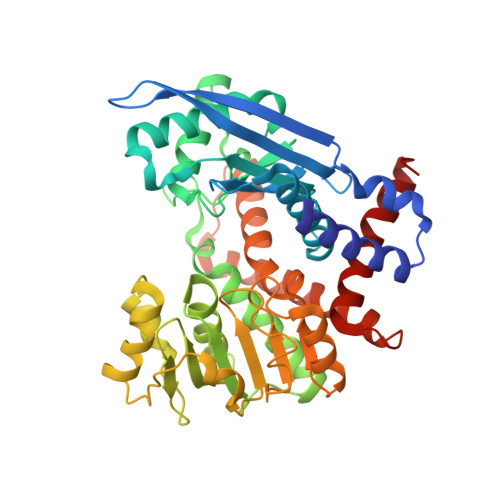
The extremely thermostable NAD-dependent glutamate dehydrogenase (NAD-GluDH) from Pyrobaculum islandicum, a member of the Crenarchaeota, was crystallized, and its 3D structure has been determined by X-ray diffraction methods. The homohexameric structure of Pb. islandicum glutamate dehydrogenase (Pis-GluDH) was solved and refined at a resolution of 2.9A with a crystallographic R-factor of 19.9% (Rfree 26.0%). The structure indicates that each subunit consists of two domains separated by a deep cleft containing an active site. The secondary structural elements and catalytically important residues of the enzyme were highly conserved among the NAD(P)-dependent GluDHs from other sources. A structural comparison of Pis-GluDH with other NAD(P)-dependent GluDHs suggests that a significant difference in the alpha8-loop-alpha9 region of this enzyme is associated with its coenzyme specificity. From the analysis of the 3D structure, hydrophobic interactions between intersubunits were found to be important features for the enzyme oligomerization. It has been reported that Pis-GluDH is highly thermostable, like the GluDH of the hyperthermophilic archaeum Pyrococcus furiosus, and the increase in the intersubunit ion pair networks is responsible for the extreme thermostability of the Pc. furiosus enzyme. However, the number of intersubunit ion pairs in the Pis-GluDH molecules is much smaller than those of the Pc. furiosus GluDH. The number of hydrophobic interactions at the intersubunit interfaces were increased and responsible for the extremely high thermostability. This indicates that the major molecular strategy for high thermostability of the GluDHs may be different for each hyperthermophile.
Organizational Affiliation:
Department of Biological Science and Technology, Faculty of Engineering, The University of Tokushima, 2-1 Minamijosanjimacho, Tokushima 770-8506, Japan.