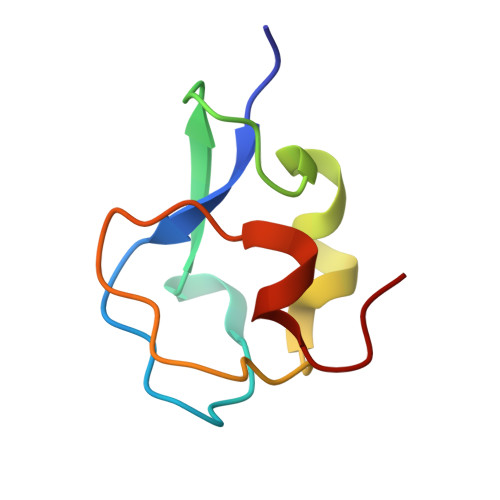
The refined crystal structure of an eel pout type III antifreeze protein RD1 at 0.62-A resolution reveals structural microheterogeneity of protein and solvation.
Ko, T.P., Robinson, H., Gao, Y.G., Cheng, C.H., DeVries, A.L., Wang, A.H.(2003) Biophys J 84: 1228-1237
- PubMed: 12547803
- DOI: https://doi.org/10.1016/S0006-3495(03)74938-8
- Primary Citation of Related Structures:
1UCS - PubMed Abstract:
RD1 is a 7-kDa globular protein from the Antarctic eel pout Lycodichthys dearborni. It belongs to type III of the four types of antifreeze proteins (AFPs) found in marine fishes living at subzero temperatures. For type III AFP, a potential ice-binding flat surface has been identified and is imbedded with side chains capable of making hydrogen bonds with a specific lattice plane on ice. So far, all crystallographic studies on type III AFPs were carried out using the Atlantic ocean pout Macrozoarces americanus as the source organism. Here we present the crystal structure of a type III AFP from a different zoarcid fish, and at an ultra-high resolution of 0.62 A. The protein fold of RD1 comprises a compact globular domain with two internal tandem motifs arranged about a pseudo-dyad symmetry. Each motif of the "pretzel fold" includes four short beta-strands and a 3(10) helix. There is a novel internal cavity of 45 A(3) surrounded by eight conserved nonpolar residues. The model contains several residues with alternate conformations, and a number of split water molecules, probably caused by alternate interactions with the protein molecule. After extensive refinement that includes hydrogen atoms, significant residual electron densities associated with the electrons of peptides and many other bonds could be visualized.
Organizational Affiliation:
Institute of Biological Chemistry, Academia Sinica, Taipei 11529, Taiwan.