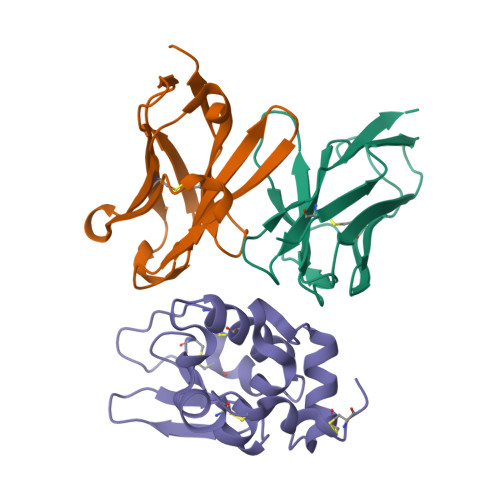
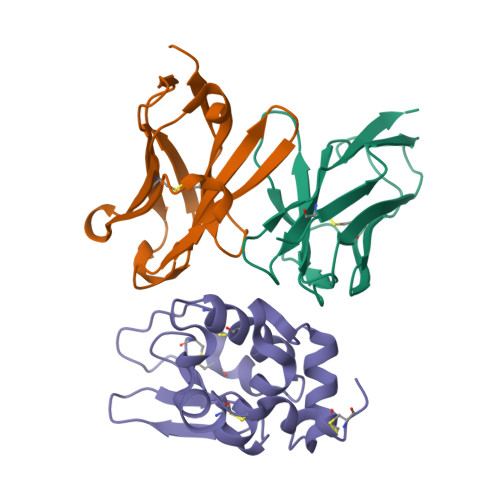
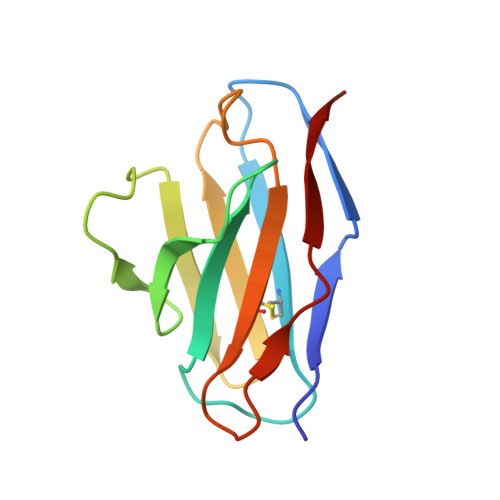
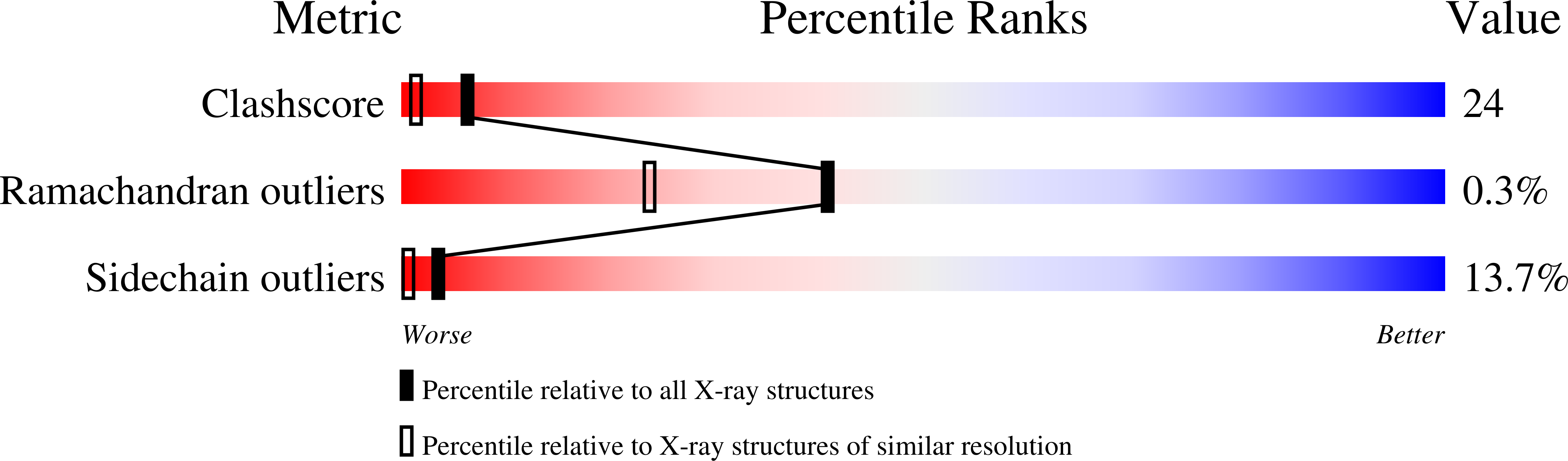Structural consequences of target epitope-directed functional alteration of an antibody. The case of anti-hen lysozyme antibody, HyHEL-10
Kumagai, I., Nishimiya, Y., Kondo, H., Tsumoto, K.(2003) J Biological Chem 278: 24929-24936
- PubMed: 12709438
- DOI: https://doi.org/10.1074/jbc.M301149200
- Primary Citation of Related Structures:
1UA6, 1UAC - PubMed Abstract:
Decreased affinity of an antibody for a mutated epitope in an antigen can be enhanced and reversed by mutations in certain antibody residues. Here we describe the crystal structures of (a) the complex between a naturally mutated proteinaceous antigen and an antibody that was mutated and selected in vitro, and (b) the complex between the normal antigen and the mutated antibody. The mutated and selected antibody recognizes essentially the same epitope as in the wild-type antibody, indicating successful target site-directed functional alteration of the antibody. In comparing the structure of the mutated antigen-mutant antibody complex with the previously established structure of the wild-type antigen-wild-type antibody complex, we found that the enhanced affinity of the mutated antibody for the mutant antigen originated not from improvements in local complementarity around the mutated sites but from subtle and critical structural changes in nonmutated sites, including an increase in variable domain interactions. Our findings indicate that only a few mutations in the antigen-binding region of an antibody can lead to some structural changes in its paratopes, emphasizing the critical roles of the plasticity of loops in the complementarity-determining region and also the importance of the plasticity of the interaction between the variable regions of immunoglobulin heavy and light chains in determining the specificity of an antibody.
Organizational Affiliation:
Department of Biomolecular Engineering, Graduate School of Engineering, Tohoku University, Aoba-yama 07, Aoba-ku, Sendai 980-8579, Japan.