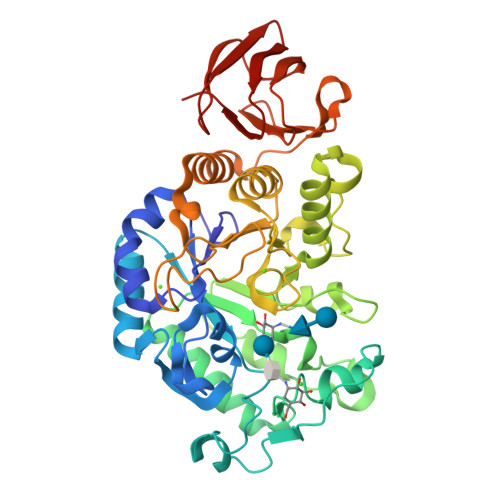
Crystal structure of Bacillus subtilis alpha-amylase in complex with acarbose
Kagawa, M., Fujimoto, Z., Momma, M., Takase, K., Mizuno, H.(2003) J Bacteriol 185: 6981-6984
- PubMed: 14617662
- DOI: https://doi.org/10.1128/JB.185.23.6981-6984.2003
- Primary Citation of Related Structures:
1UA7 - PubMed Abstract:
The crystal structure of Bacillus subtilis alpha-amylase, in complex with the pseudotetrasaccharide inhibitor acarbose, revealed an hexasaccharide in the active site as a result of transglycosylation. After comparison with the known structure of the catalytic-site mutant complexed with the native substrate maltopentaose, it is suggested that the present structure represents a mimic intermediate in the initial stage of the catalytic process.
Organizational Affiliation:
Department of Biochemistry, National Institute of Agrobiological Sciences, Tsukuba, Ibaraki 305-8602, Japan.