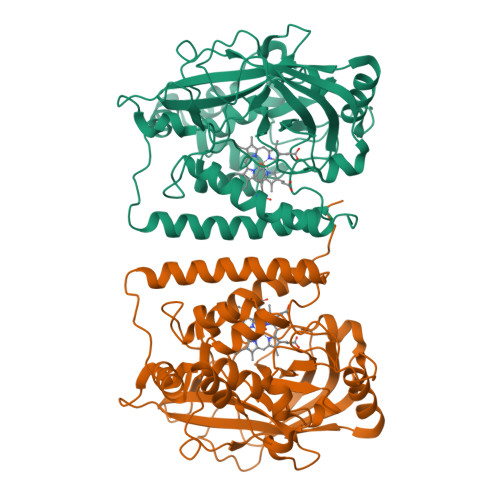
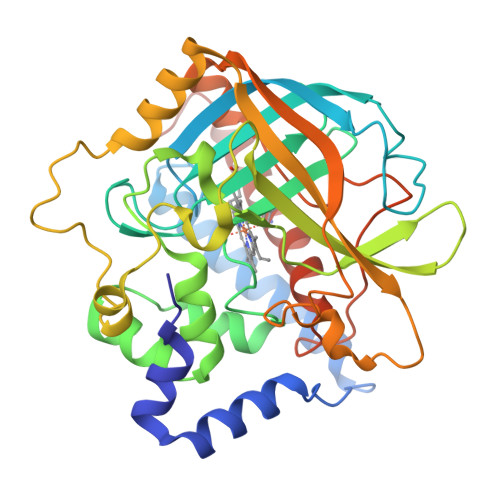
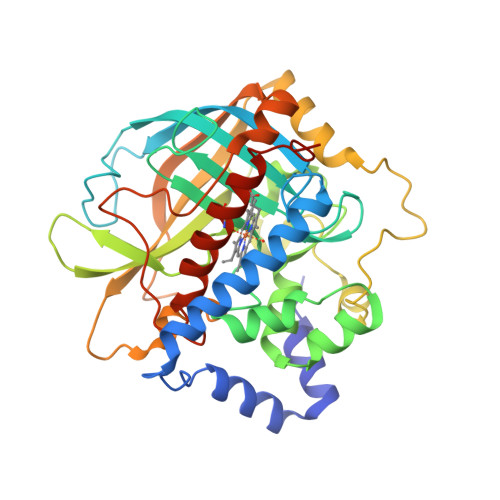
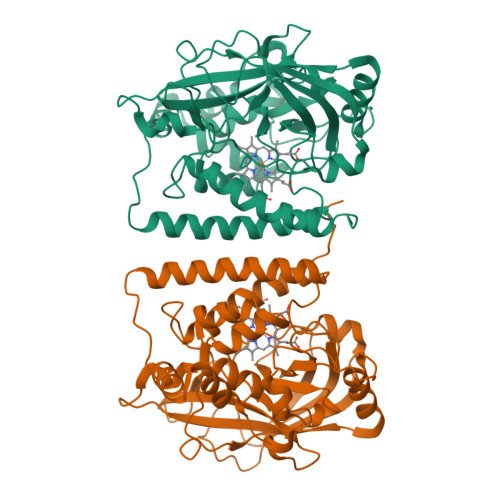
The structure of coral allene oxide synthase reveals a catalase adapted for metabolism of a fatty acid hydroperoxide.
Oldham, M.L., Brash, A.R., Newcomer, M.E.(2005) Proc Natl Acad Sci U S A 102: 297-302
- PubMed: 15625113
- DOI: https://doi.org/10.1073/pnas.0406352102
- Primary Citation of Related Structures:
1U5U - PubMed Abstract:
8R-Lipoxygenase and allene oxide synthase (AOS) are parts of a naturally occurring fusion protein from the coral Plexaura homomalla. AOS catalyses the production of an unstable epoxide (an allene oxide) from the fatty acid hydroperoxide generated by the lipoxygenase activity. Here, we report the structure of the AOS domain and its striking structural homology to catalase. Whereas nominal sequence identity between the enzymes had been previously described, the extent of structural homology observed was not anticipated, given that this enzyme activity had been exclusively associated with the P450 superfamily, and conservation of a catalase fold without catalase activity is unprecedented. Whereas the heme environment is largely conserved, the AOS heme is planar and the distal histidine is flanked by two hydrogen-bonding residues. These critical differences likely facilitate the switch from a catalatic activity to that of a fatty acid hydroperoxidase.
Organizational Affiliation:
Department of Biological Sciences, Louisiana State University, Baton Rouge, LA 70803, USA.